This document discusses challenges in predictive data analytics for biopharmaceutical process and product development. It notes that process and analytical variability exists between laboratory, pilot, and production scales. Predictive modeling and data fusion techniques can help address this variability and improve quality across scales by integrating product and process development. Case studies will demonstrate these approaches for fermentation processes.






![Process and Analytical Variability Challenges
‘Product Quality’ across lab, pilot & production scales
(Staffan Folestad AstraZeneca, APACT09)
Spectra from different probes
show distinct Inter-probe variability
Position 1
P 2
P3
P4
-20
-10
0
10
20
t[2]
t[1]
•P1-2225.0
•P1-
•P1-2303.0•P1-2322.0
•P1-2343.0
•P1-2401.0
•P1-2420.0
•P1-2552.0
•P1-2611.0
•P1-2534.0•P1-2648.0
•P1-2710.0
•P1-2728.0
•P1-2629.0
•P1-2842.0
•P1-2919.0
•P1-2746.0•P1-2805.0
•P1-2823.0•P1-2900.0
•P1-3014.0
•P1-3245.0•P1-3131.0
•P1-3726.0
•P1-3341.0
•P1-3418.0
•P1-2956.0
•P1-3304.0
•P1-3150.0
•P1-3208.0
•P1-3840.0•P1-3744.0
•P1-3803.0
•P1-4628.0
•P1-3437.0
•P1-3455.0
•P1-3514.0
•P1-3400.0
•P1-3708.0
•P1-3227.0
•P1-4225.0
•P1-4244.0
•P1-3859.0
•P1-4015.0
•P1-4609.0
•P1-4646.0
•P1-4514.0
•P1-4551.0
•P1-4819.0
•P1-4837.0
•P1-4705.0
•P1-4723.0
•P1-4742.0
•P1-3532.0
•P1-4532.0
•P1-4800.0
•P2-5400.0
•P2-5537.0
•P2-5746.0
•P2-5708.0
•P2-5919.0
•P2-5937.0
•P2-5900.0
•P2-0033.0
•P2-5805.0•P2-5507.0
•P2-5440.0
•P2-5420.0
•P2-5610.0
•P2-5727.0
•P2-5629.0
•P2-5649.0
•P2-0150.0
•P2-0245.0
•P2-2053.0
•P2-0707.0
•P2-0630.0
•P2-0612.0
•P2-0322.0
•P2-0208.0
•P2-0227.0
•P2-0437.0
•P2-0418.0
•P2-1440.0•P2-1147.0
•P2-2206.0
•P2-2111.0
•P2-2225.0
•P2-2342.0•P2-2244.0•P2-1806.0
•P2-1727.0•P2-1939.0
•P2-0649.0
•P2-0553.0•P2-0725.0
•P2-0839.0
•P2-0821.0
•P2-0744.0•P2-0803.0
•P2-0534.0
•P2-0304.0
•P2-0359.0
•P2-1421.0
•P2-1206.0•P2-1129.0
•P2-1650.0•P2-2148.0
•P2-2129.0
•P2-2400.0•P2-2323.0
•P2-2305.0•P2-2034.0
•P2-1825.0
•P2-1748.0
•P2-1708.0
•P2-1843.0
•P2-1957.0•P2-2015.0
•P2-1902.0
•P2-1920.0
•P2-0916.0
•P2-0858.0
•P4-2006.0
•P4-2348.0
•P4-1849.0
•P4-1658.0
•P4-1409.0•P4-1427.0
•P4-1331.0
•P4-1254.0
•P4-1313.0•P4-1603.0•P4-1621.0
•P4-1640.0
•P4-3040.0
•P4-2311.0
•P4-2253.0•P4-2157.0
•P4-2216.0
•P4-2234.0
•P4-2600.0
•P4-2427.0
•P4-2446.0
•P4-1217.0
•P4-1831.0
•P4-1350.0
•P4-1236.0
•P4-1544.0
•P4-2945.0
•P4-2849.0
•P4-2908.0
•P4-2926.0
•P4-2619.0
•P4-2330.0
•P4-2523.0
•P4-2542.0
•P4-2504.0•P4-2409.0
•P3-4438.0
•P3-5058.0•P3-5019.0
•P3-4941.0
•P3-5232.0
•P3-5154.0
•P3-5117.0
•P3-5135.0
•P3-4650.0
•P3-4631.0•P3-4845.0
•P4-0158.0
•P4-0220.0•P4-0238.0
•P3-4242.0
•P3-4516.0
•P3-4535.0
•P3-4457.0
•P3-4127.0
•P3-4050.0
•P3-5038.0
•P3-5000.0
•P3-5213.0
•P3-4922.0•P3-4708.0
•P3-4730.0
•P3-4612.0
•P3-3137.0
•P3-3640.0
•P3-3701.0
•P3-3621.0
•P3-3835.0
•P3-3446.0
•P3-3505.0
•P3-3427.0
•P3-3350.0
•P3-3408.0
•P4-0026.0
•P4-0140.0
•P4-0044.0
•P3-4340.0
•P3-4321.0•P3-4301.0
•P3-4420.0
•P3-4109.0
•P3-4554.0
•P3-3002.0
•P3-2921.0
•P3-3118.0
•P3-3059.0
•P3-3020.0
•P3-3039.0
•P3-3155.0
•P3-3544.0
•P3-3603.0•P3-3816.0
•P3-3233.0
•P4-0007.0•P4-5912.0
•P3-4401.0
•P3-2940.0
•P3-2844.0
•P3-2902.0
•P4-5949.0
•P4-5931.0
•P4-5854.0
•P3-2748.0
•P3-2806.0
•P3-2729.0
•P3-2710.0
•P4-5835.0
•P3-2651.0
-100 -80 -60 -40 -20 0 20 40 60 80 100
P2
P1
Position 1
P 2
P3
P4
-20
-10
0
10
20
t[2]
t[1]
•P1-2225.0
•P1-
•P1-2303.0•P1-2322.0
•P1-2343.0
•P1-2401.0
•P1-2420.0
•P1-2552.0
•P1-2611.0
•P1-2534.0•P1-2648.0
•P1-2710.0
•P1-2728.0
•P1-2629.0
•P1-2842.0
•P1-2919.0
•P1-2746.0•P1-2805.0
•P1-2823.0•P1-2900.0
•P1-3014.0
•P1-3245.0•P1-3131.0
•P1-3726.0
•P1-3341.0
•P1-3418.0
•P1-2956.0
•P1-3304.0
•P1-3150.0
•P1-3208.0
•P1-3840.0•P1-3744.0
•P1-3803.0
•P1-4628.0
•P1-3437.0
•P1-3455.0
•P1-3514.0
•P1-3400.0
•P1-3708.0
•P1-3227.0
•P1-4225.0
•P1-4244.0
•P1-3859.0
•P1-4015.0
•P1-4609.0
•P1-4646.0
•P1-4514.0
•P1-4551.0
•P1-4819.0
•P1-4837.0
•P1-4705.0
•P1-4723.0
•P1-4742.0
•P1-3532.0
•P1-4532.0
•P1-4800.0
•P2-5400.0
•P2-5537.0
•P2-5746.0
•P2-5708.0
•P2-5919.0
•P2-5937.0
•P2-5900.0
•P2-0033.0
•P2-5805.0•P2-5507.0
•P2-5440.0
•P2-5420.0
•P2-5610.0
•P2-5727.0
•P2-5629.0
•P2-5649.0
•P2-0150.0
•P2-0245.0
•P2-2053.0
•P2-0707.0
•P2-0630.0
•P2-0612.0
•P2-0322.0
•P2-0208.0
•P2-0227.0
•P2-0437.0
•P2-0418.0
•P2-1440.0•P2-1147.0
•P2-2206.0
•P2-2111.0
•P2-2225.0
•P2-2342.0•P2-2244.0•P2-1806.0
•P2-1727.0•P2-1939.0
•P2-0649.0
•P2-0553.0•P2-0725.0
•P2-0839.0
•P2-0821.0
•P2-0744.0•P2-0803.0
•P2-0534.0
•P2-0304.0
•P2-0359.0
•P2-1421.0
•P2-1206.0•P2-1129.0
•P2-1650.0•P2-2148.0
•P2-2129.0
•P2-2400.0•P2-2323.0
•P2-2305.0•P2-2034.0
•P2-1825.0
•P2-1748.0
•P2-1708.0
•P2-1843.0
•P2-1957.0•P2-2015.0
•P2-1902.0
•P2-1920.0
•P2-0916.0
•P2-0858.0
•P4-2006.0
•P4-2348.0
•P4-1849.0
•P4-1658.0
•P4-1409.0•P4-1427.0
•P4-1331.0
•P4-1254.0
•P4-1313.0•P4-1603.0•P4-1621.0
•P4-1640.0
•P4-3040.0
•P4-2311.0
•P4-2253.0•P4-2157.0
•P4-2216.0
•P4-2234.0
•P4-2600.0
•P4-2427.0
•P4-2446.0
•P4-1217.0
•P4-1831.0
•P4-1350.0
•P4-1236.0
•P4-1544.0
•P4-2945.0
•P4-2849.0
•P4-2908.0
•P4-2926.0
•P4-2619.0
•P4-2330.0
•P4-2523.0
•P4-2542.0
•P4-2504.0•P4-2409.0
•P3-4438.0
•P3-5058.0•P3-5019.0
•P3-4941.0
•P3-5232.0
•P3-5154.0
•P3-5117.0
•P3-5135.0
•P3-4650.0
•P3-4631.0•P3-4845.0
•P4-0158.0
•P4-0220.0•P4-0238.0
•P3-4242.0
•P3-4516.0
•P3-4535.0
•P3-4457.0
•P3-4127.0
•P3-4050.0
•P3-5038.0
•P3-5000.0
•P3-5213.0
•P3-4922.0•P3-4708.0
•P3-4730.0
•P3-4612.0
•P3-3137.0
•P3-3640.0
•P3-3701.0
•P3-3621.0
•P3-3835.0
•P3-3446.0
•P3-3505.0
•P3-3427.0
•P3-3350.0
•P3-3408.0
•P4-0026.0
•P4-0140.0
•P4-0044.0
•P3-4340.0
•P3-4321.0•P3-4301.0
•P3-4420.0
•P3-4109.0
•P3-4554.0
•P3-3002.0
•P3-2921.0
•P3-3118.0
•P3-3059.0
•P3-3020.0
•P3-3039.0
•P3-3155.0
•P3-3544.0
•P3-3603.0•P3-3816.0
•P3-3233.0
•P4-0007.0•P4-5912.0
•P3-4401.0
•P3-2940.0
•P3-2844.0
•P3-2902.0
•P4-5949.0
•P4-5931.0
•P4-5854.0
•P3-2748.0
•P3-2806.0
•P3-2729.0
•P3-2710.0
•P4-5835.0
•P3-2651.0
-100 -80 -60 -40 -20 0 20 40 60 80 100
P2
P1
Sensing space direction
PCA of spectra collected for over 1 hr
Courtesy R O’Kennedy et al (GSK & Univ Strathclyde)
Agitator
L1
L2
L3
L4
Probe Location
L2
L3
L4
Probe Location
‘Identical Reactors – Different heat-
transfer characteristics ?’
16m3
35m3
24m3
24m3
24m3
Are
‘Iden)cal
Disposables’
‘Iden)cal?’](https://image.slidesharecdn.com/julianmorisnewcastleppt-160308123212/85/Newcastle-BILS-2016-7-320.jpg)








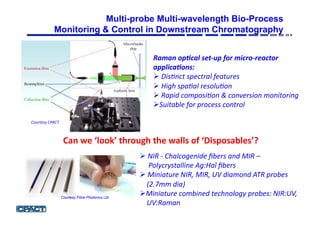





![■ The process under study was an industrial pilot-plant scale
streptomyces fermentation involving a seed stage and a production
stage.
■ Biomass was grown in the seed stage and then transferred into the
final fed batch stage for the production of the product lasting
approximately 140hrs.
■ Two sets of fermentation experiments were carried out. The first set
comprised seven batches (calibration batches); the second set was
made up of six batches (test batches).
■ The seven calibration batches were run under similar conditions, but
natural variation provided some degree of variability.
■ For the test batches, the runs were carried out under different
environmental conditions (pH and temperature), feed rates (sugar
feed) and feed amounts (oil feed).
Coping with Process Variation [Courtesy GSK]](https://image.slidesharecdn.com/julianmorisnewcastleppt-160308123212/85/Newcastle-BILS-2016-23-320.jpg)
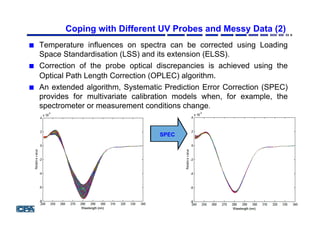
![Prediction of Product Concentration [Courtesy GSK]
PLS calibration built using the spectra of the
samples from primary instrument.
Global PLS using the spectra samples from the primary instrument
and six standardisation samples from the secondary instrument
Blue circles - product
concentration by off-line assay
Systematic Prediction Error Correction Algorithm
Stretomyces Fermentation](https://image.slidesharecdn.com/julianmorisnewcastleppt-160308123212/85/Newcastle-BILS-2016-24-320.jpg)