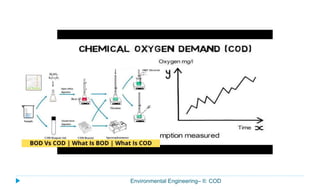
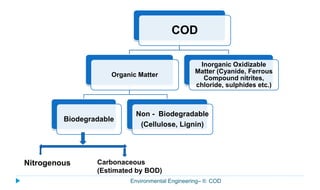
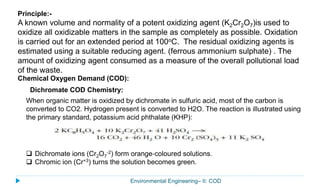
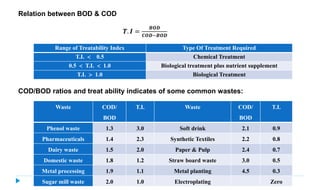
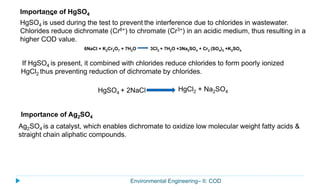
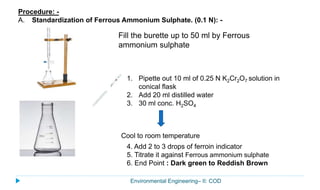
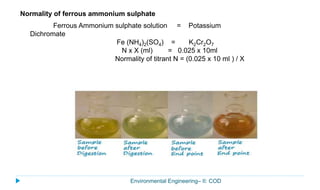
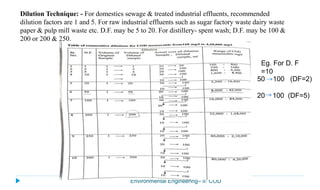
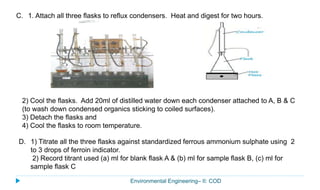
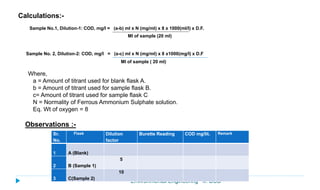
This document provides information about the chemical oxygen demand (COD) test for measuring organic matter in wastewater. It discusses that COD measures the oxygen required to chemically oxidize organic material using potassium dichromate and sulfuric acid. COD and BOD both measure how much oxygen water will consume, but COD can oxidize more material so values are higher than BOD. The document outlines the COD test procedure and calculations for determining COD levels in wastewater samples. It also discusses standards, sources of BOD and COD, and limitations of the COD test.