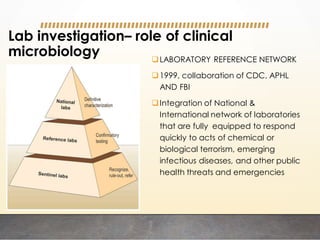
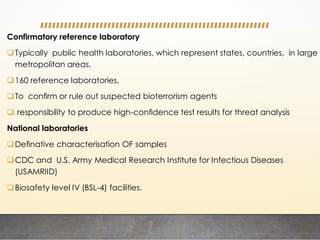
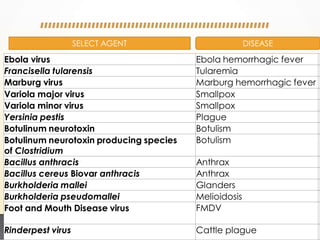
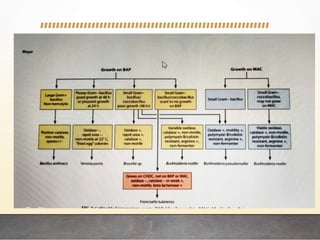
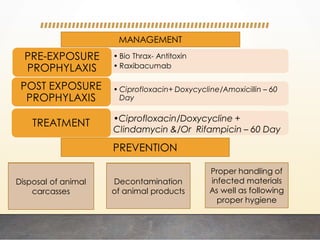
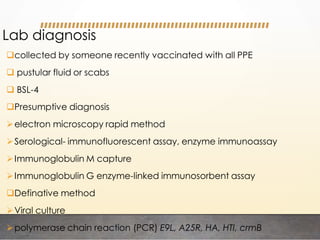
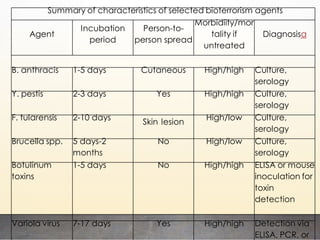
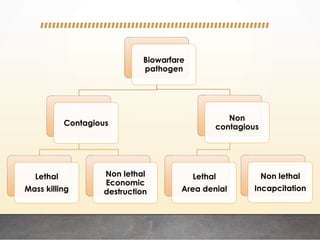
This document discusses bioterrorism and countermeasures against it. It defines bioterrorism and describes ideal biological agents for bioweapons. It outlines the categories of biological agents that pose threats from categories A to C. It discusses the history of bioweapons use from ancient times to the 21st century. It also describes India's defenses against bioterrorism, including surveillance programs, laboratories, and medical preparedness. Key response measures involve deterring attacks, preventing access to pathogens, rapidly diagnosing outbreaks, and treating affected populations.









![Category A
Anthrax (Bacillus anthracis)
Botulism (Clostridium botulinum toxin)
Plague (Yersinia pestis)
Smallpox (variola major)
Tularemia (Francisella tularensis)
Viral hemorrhagic fevers (e.g., Ebola, Marburg, Lassa, Machupo])](https://image.slidesharecdn.com/bioterrorismskg-220929052142-af04a35f/85/BIOTERRORISM-pdf-11-320.jpg)
![Category B
Brucellosis (Brucella species)
Epsilon toxin of Clostridium perfringens
Food safety threats (e.g., Salmonella species, Escherichia coli O157:H7,
Shigella)
Glanders (Burkholderia mallei)
Melioidosis (Burkholderia pseudomallei)
Psittacosis (Chlamydia psittaci)
Q fever (Coxiella burnetii)
Ricin toxin from Ricinus communis (castor beans)
Staphylococcal enterotoxin B
Typhus fever (Rickettsia prowazekii)
Viral encephalitis (alphaviruses [e.g., Venezuelan equine encephalitis,
eastern equine encephalitis, western equine encephalitis])
Water safety threats (e.g., Vibrio cholerae, Cryptosporidium parvum)](https://image.slidesharecdn.com/bioterrorismskg-220929052142-af04a35f/85/BIOTERRORISM-pdf-12-320.jpg)