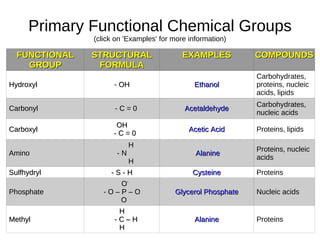
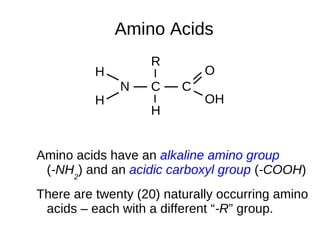
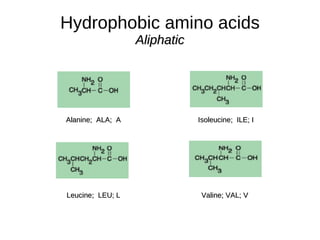
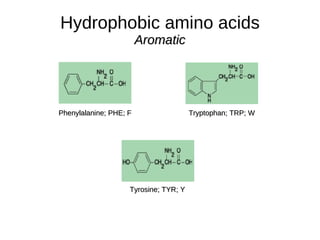
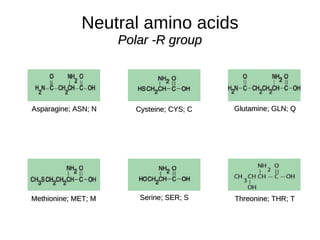
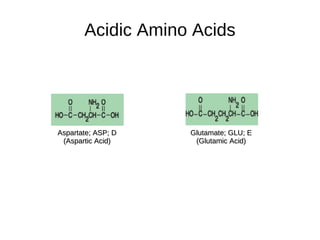
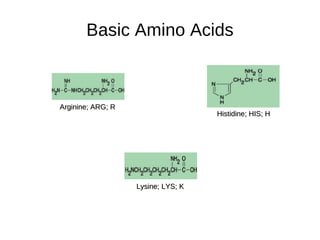
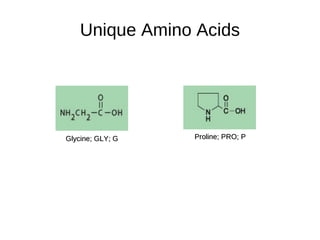
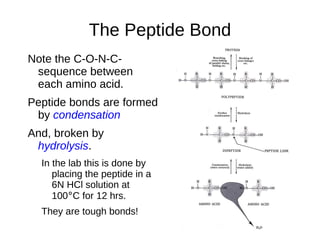
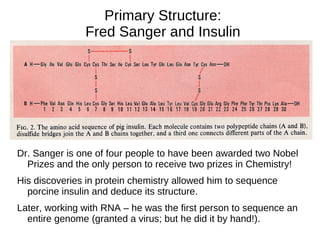
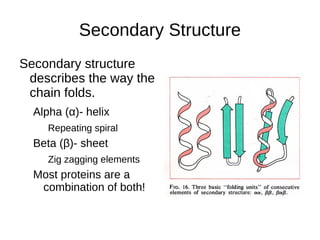
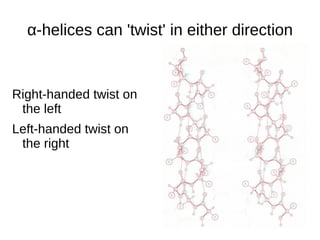
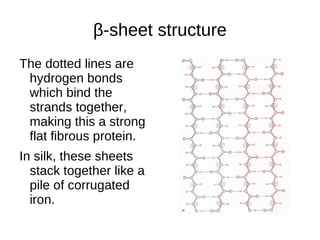
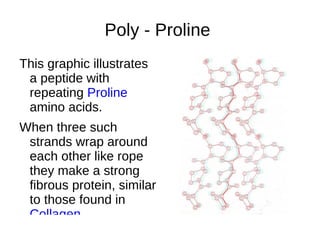
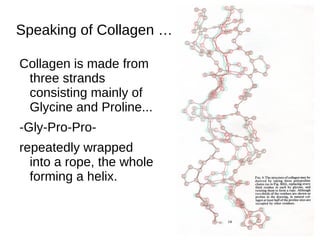
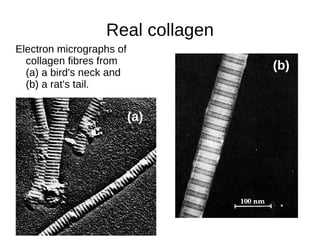
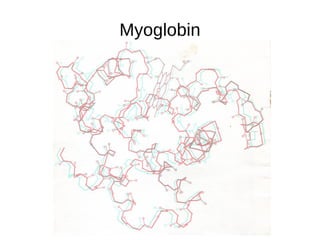
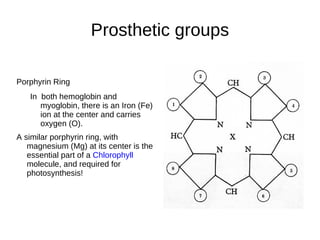
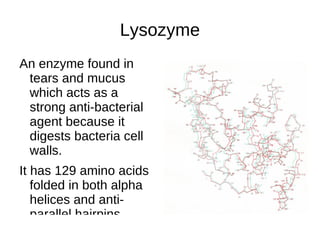
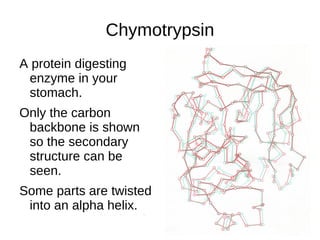
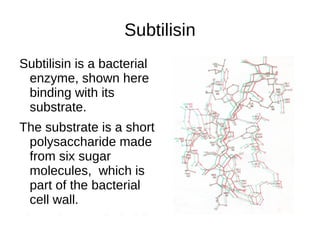
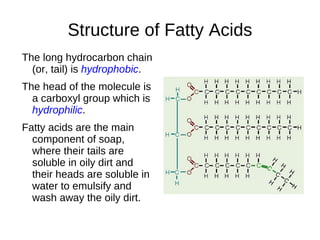
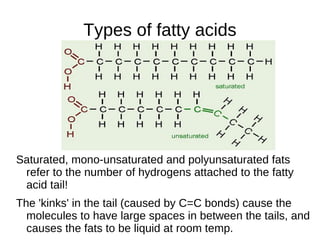
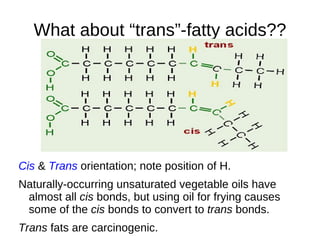
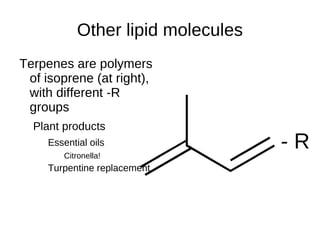
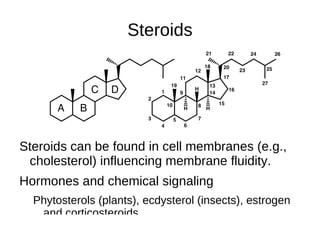
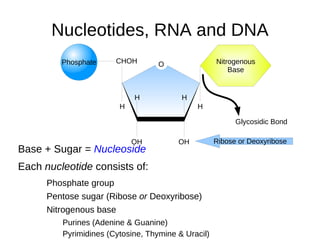
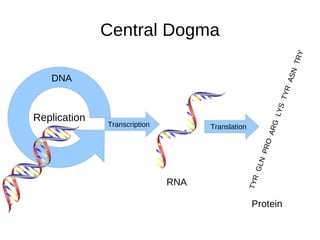
This document outlines the chemistry of life, focusing on biochemistry and the major macromolecules: carbohydrates, proteins, lipids, and nucleic acids. It details the structure and function of amino acids and proteins, along with the formation and significance of peptide bonds, secondary structures, and protein types. Additionally, it covers fatty acids, lipids, and nucleotides, along with the central dogma of molecular biology involving DNA, RNA, and proteins.