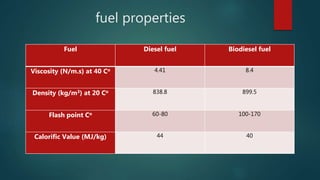
This document discusses biodiesel production and properties. It begins by defining biodiesel as an alternative fuel made from vegetable oils or animal fats through a process called transesterification. This process converts triglycerides into fatty acid alkyl esters and glycerin. The document then covers biodiesel's production method using waste cooking oil, its fuel properties like higher flash point but lower energy density compared to diesel, lower exhaust emissions but also lower power output. Lastly, it addresses biodiesel storage and transportation challenges like its solvent properties and tendency to gel at higher temperatures than petrodiesel.