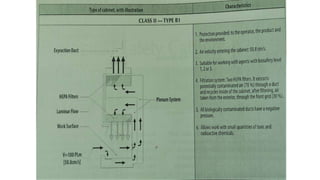
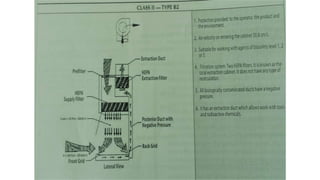
The document provides an overview of biological safety cabinets, detailing their purpose of protecting the user, environment, and samples from infectious materials. It discusses operational principles, installation requirements, routine maintenance, and troubleshooting procedures necessary for ensuring the functionality of these cabinets in laboratory settings. Additionally, it outlines procedures for specialized maintenance tasks such as motor changes and HEPA filter replacements.