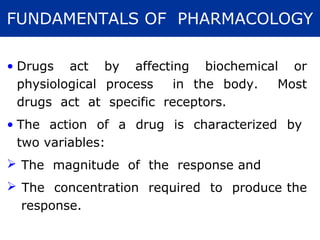
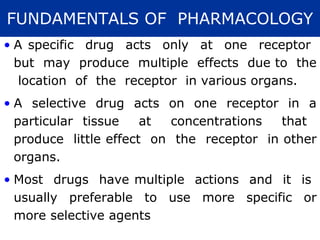
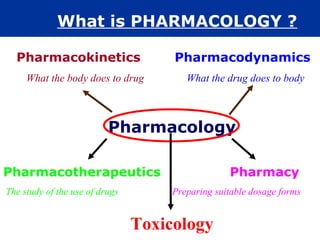
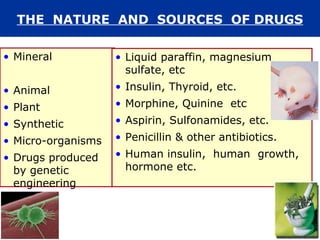
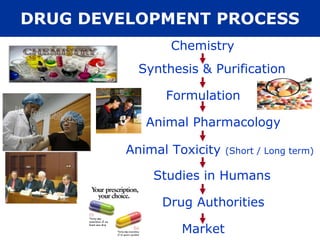
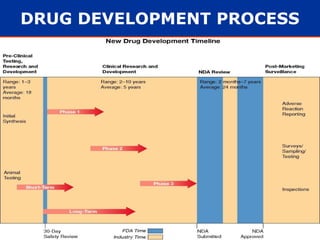
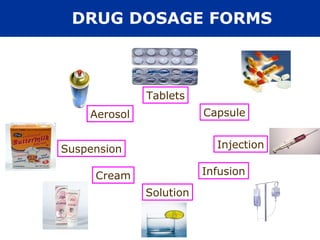
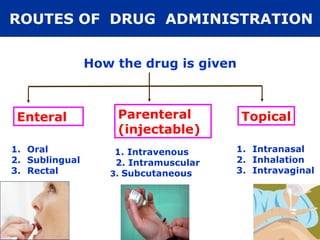
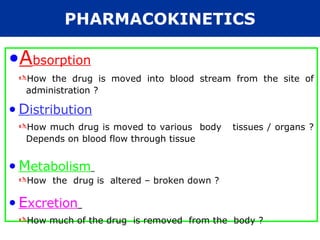
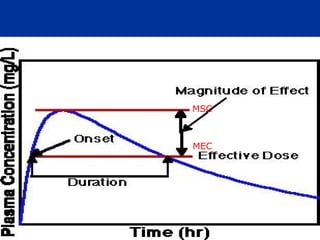
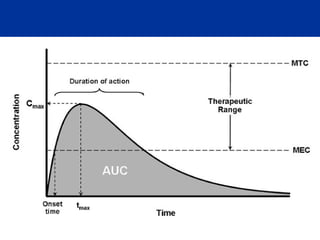
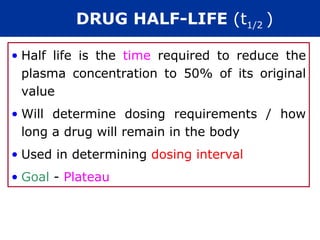
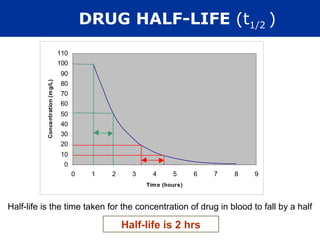
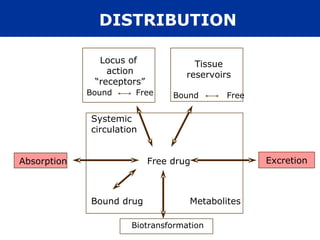
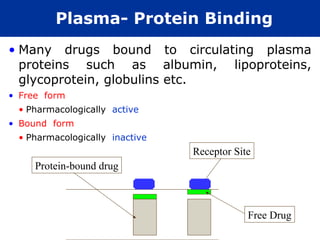
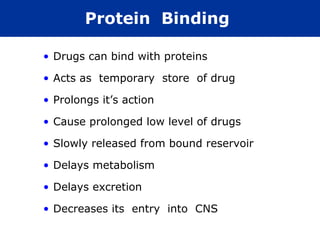
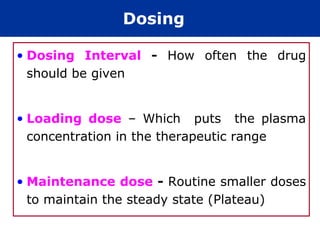
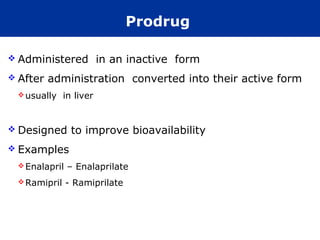
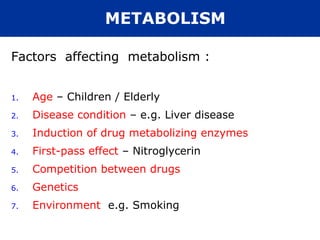
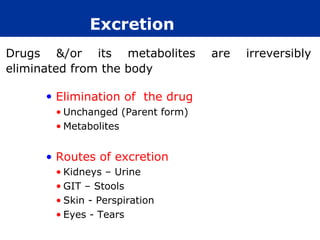
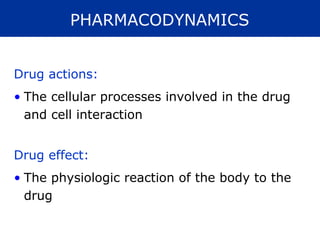
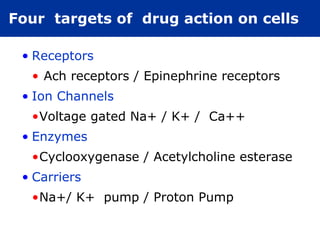
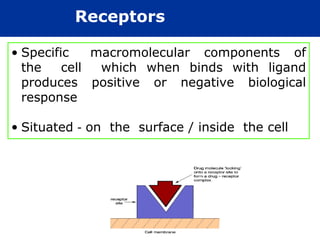
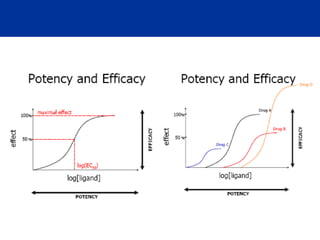
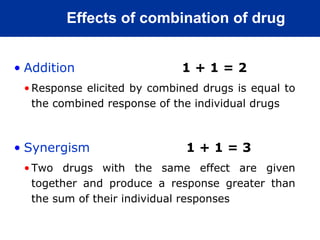
The document provides information about pharmacology and related topics. It discusses the definition of pharmacology as the study of drugs and their actions on the body. It also covers key concepts such as pharmacokinetics, pharmacodynamics, drug dosage forms, routes of administration, absorption, distribution, metabolism, excretion, and factors that influence drug response.





































![FUNDAMENTALS OF PHARMACOLOGY
• The rational pharmacological treatment of
any patient requires adequate knowledge
about :
The disease process,
Pharmacodynamic properties of the
drug(s) selected, and
The individual’s handling of the drug(s)
[pharmacokinetics].](https://image.slidesharecdn.com/pharmacology-120806022529-phpapp01/85/Pharmacology-85-320.jpg)