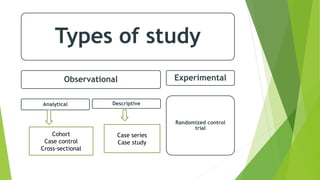
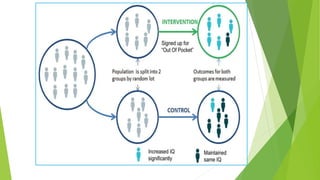
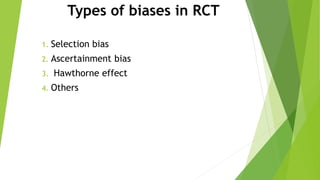
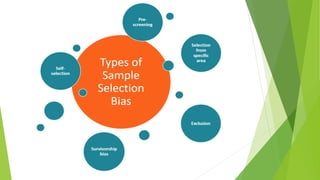
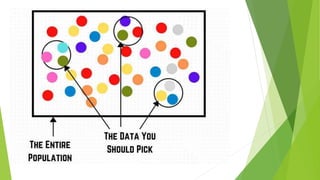
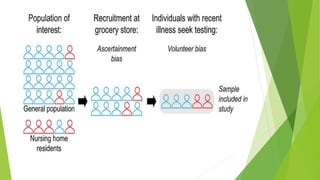
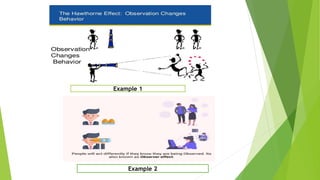
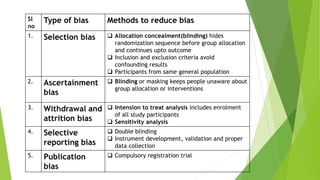
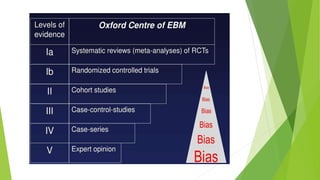
The document discusses biases in randomized control trials (RCTs) and their potential effects on study outcomes. It outlines various types of biases such as selection and ascertainment bias, along with methods to minimize their impact, including allocation concealment and blinding. The importance of assessing bias risk and maintaining high-quality evidence in trials is emphasized.