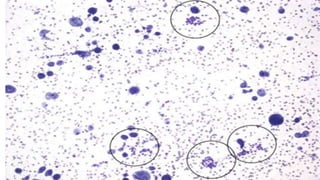
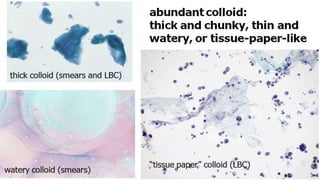
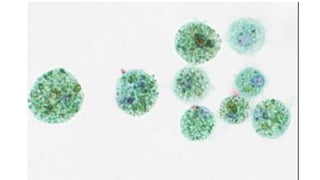
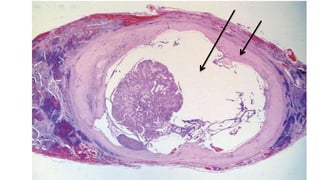
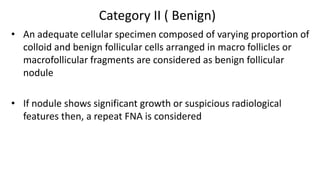
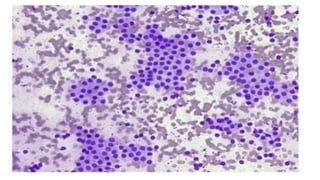
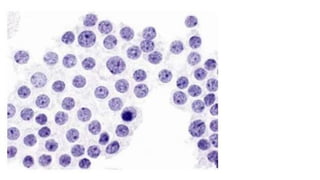
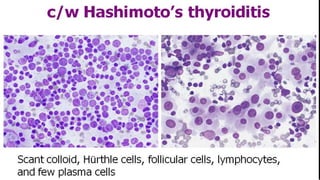
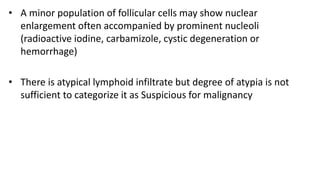
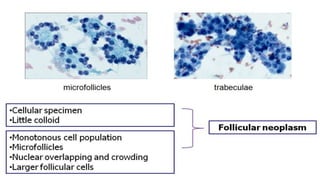
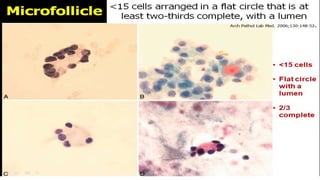
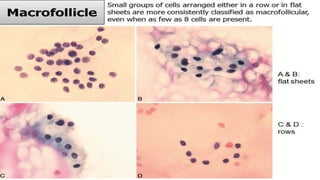
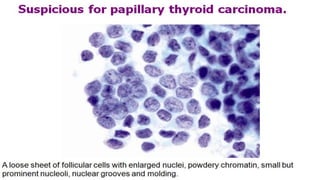
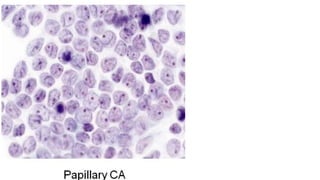
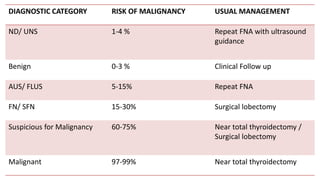
The document summarizes the recommendations from an October 2007 meeting at the National Cancer Institute to develop a uniform reporting system for thyroid fine needle aspiration biopsies. It outlines six diagnostic categories with implied cancer risks and descriptions. The categories include non-diagnostic or unsatisfactory, benign, atypia of undetermined significance or follicular lesion of undetermined significance, follicular neoplasm or suspicious for follicular neoplasm, suspicious for malignancy, and malignant. Criteria for each category are provided along with typical clinical management based on the cancer risk implication of each category.