The document provides information on batteries, including:
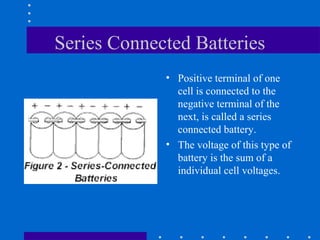
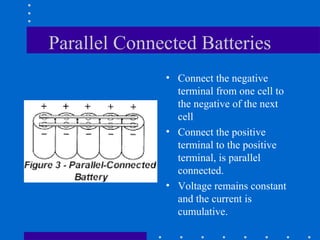
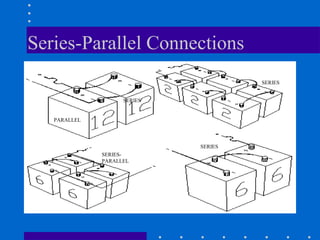
1) Batteries convert chemical energy into electrical energy through reversible chemical reactions and can be recharged by passing current in the opposite direction of discharge.
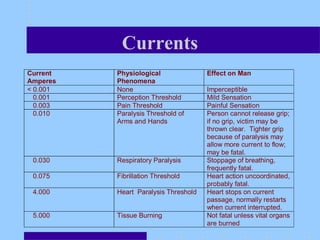
2) Batteries contain hazardous materials like sulfuric acid and lead that can cause burns, nerve damage, and other health issues if exposed.
3) Primary batteries convert chemical energy directly while secondary batteries must be charged first before use and can be recharged multiple times.