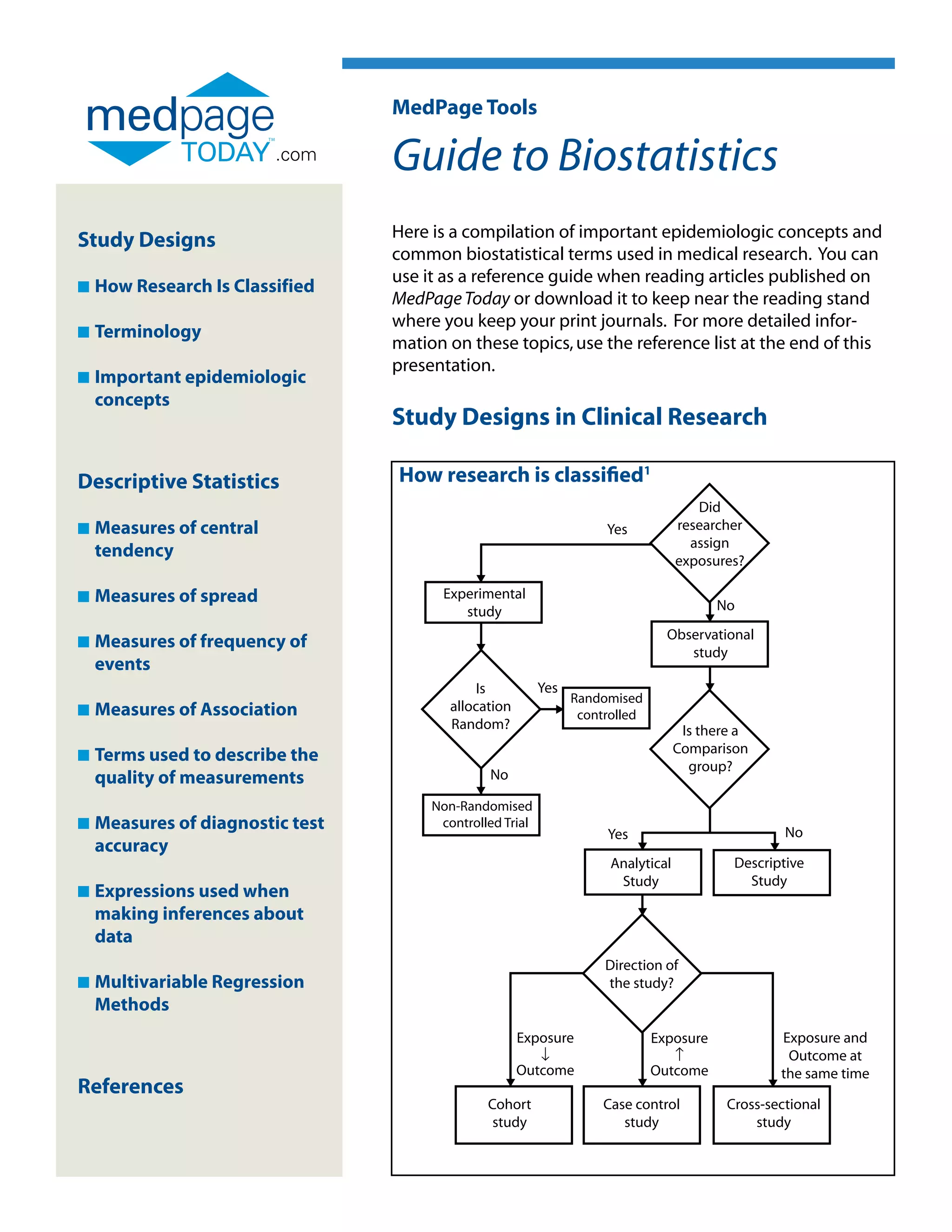
This document provides a guide to important concepts in biostatistics and epidemiology used in medical research. It defines different types of study designs including experimental, observational, cohort, case-control and cross-sectional studies. It also defines key terminology used in these fields such as bias, confounding, measures of central tendency, measures of association like relative risk and odds ratio, and measures used to describe the quality of diagnostic tests and measurements. The guide is intended to help readers understand concepts in research articles on medical topics.