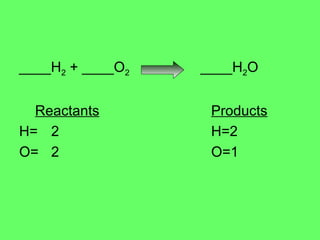
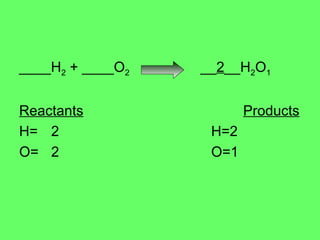
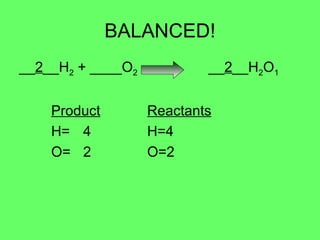
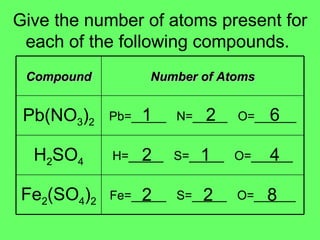
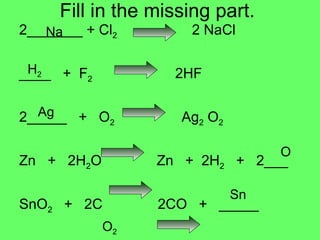
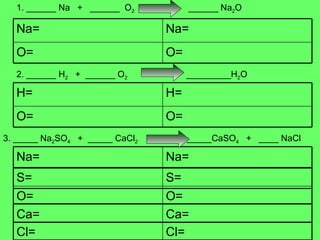
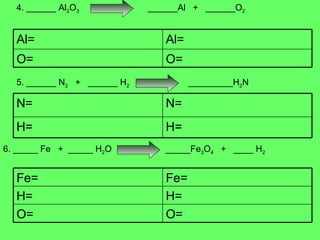
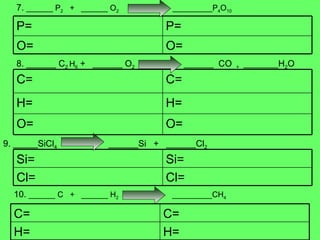
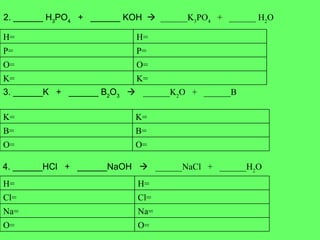
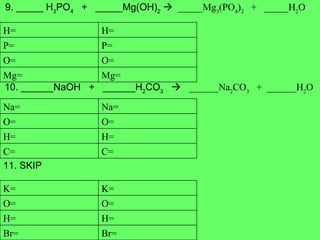
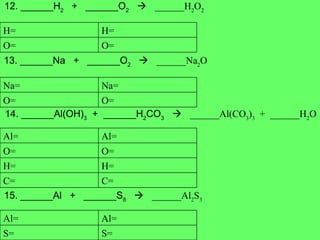
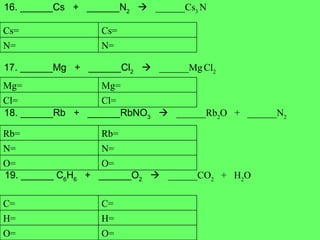
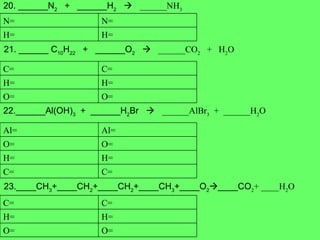
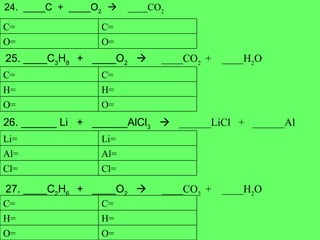
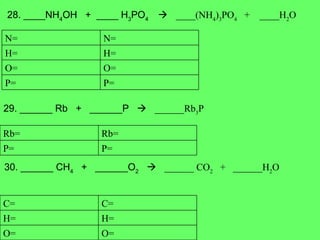
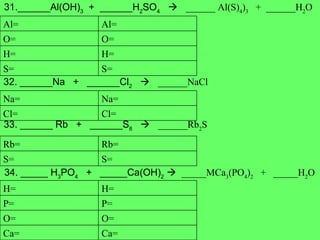
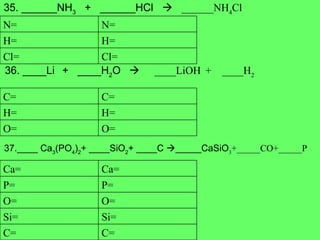
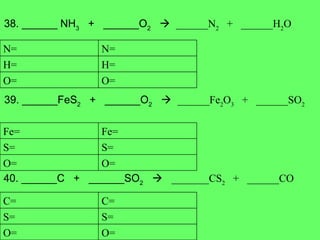
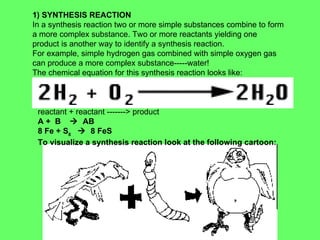
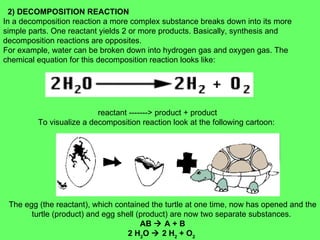
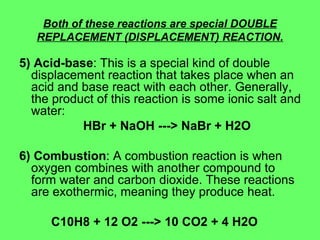
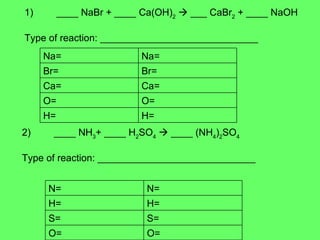
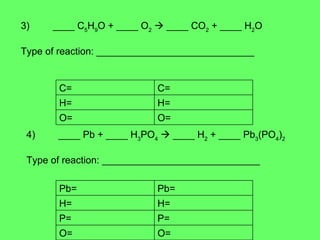
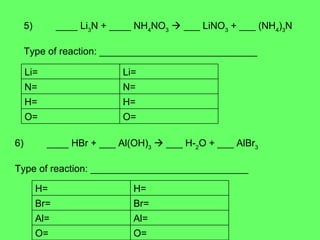
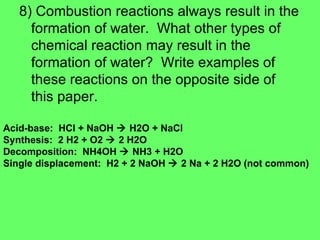
The document discusses balancing chemical equations and types of chemical reactions. It defines six types of chemical reactions: synthesis, decomposition, single replacement, double replacement, acid-base, and combustion reactions. It provides examples of each type of reaction and their balanced chemical equations. Worksheets are included for the reader to balance additional example reactions and identify the reaction type.