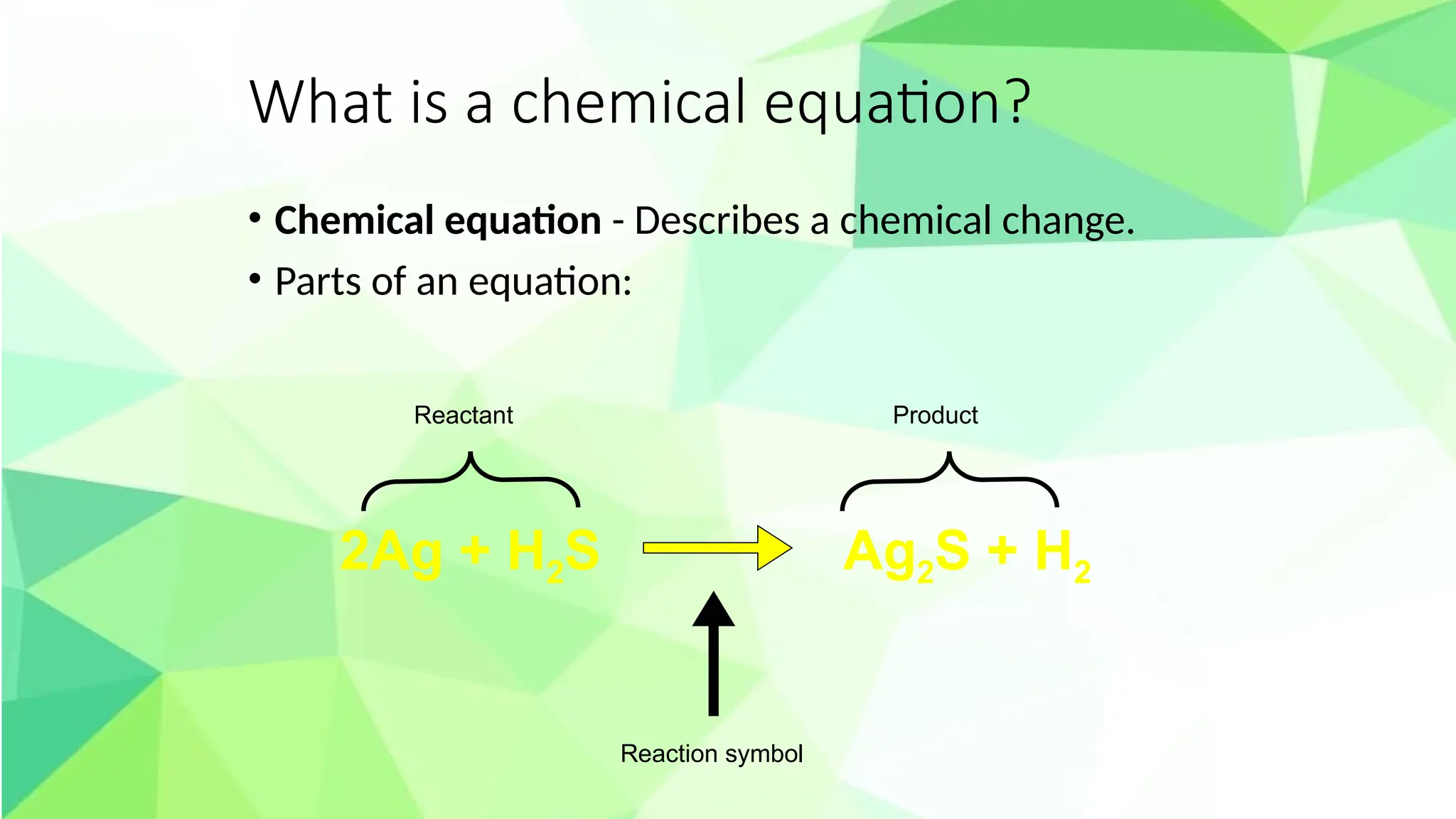
The document outlines a Grade 10 science lesson focused on chemical equations and reactions. It includes activities for identifying chemical symbols, defining chemical equations, and balancing equations while emphasizing the law of conservation of mass. Students are expected to engage in various activities to apply these concepts, culminating in a project presenting a chemical reaction relevant to biology and industry.