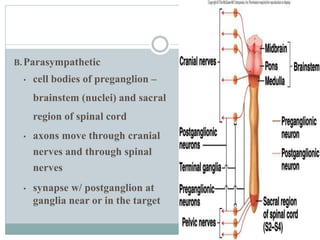
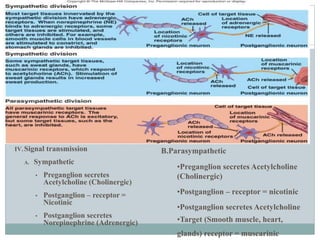
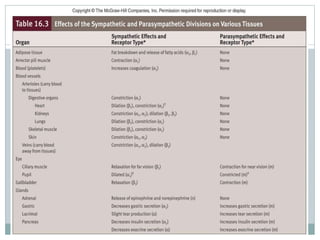
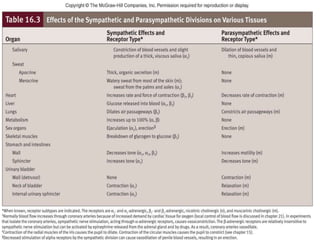
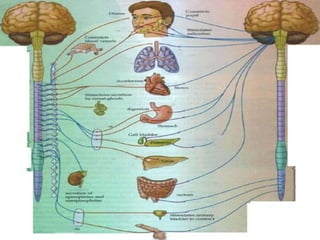
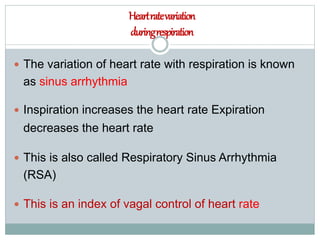
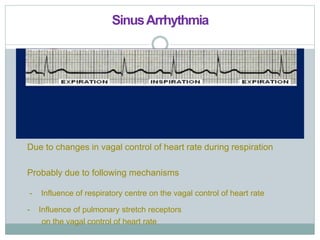
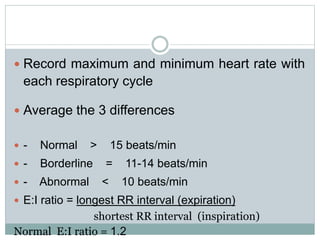
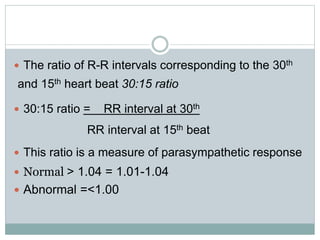
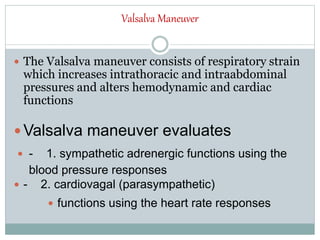
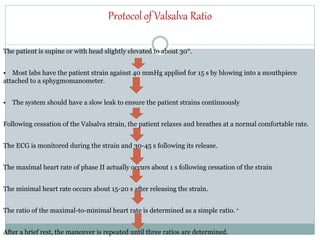
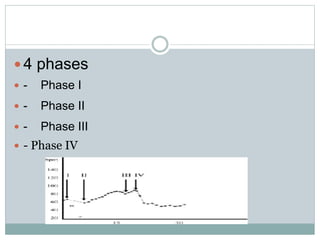
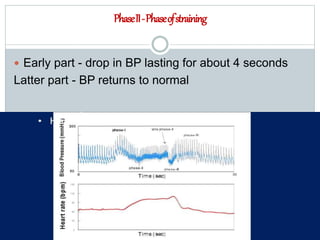
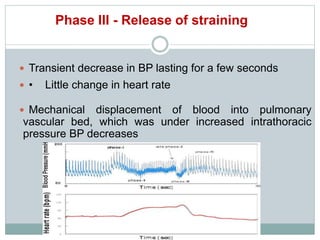
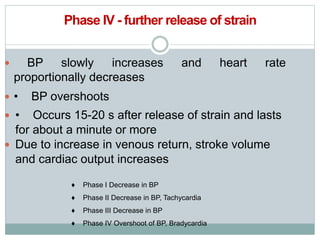
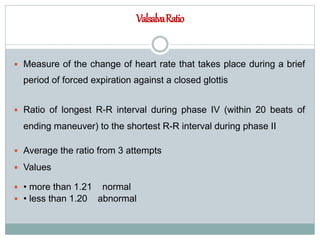
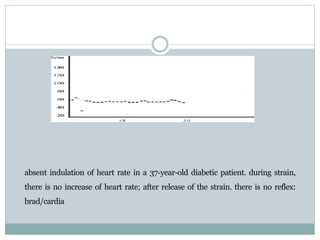
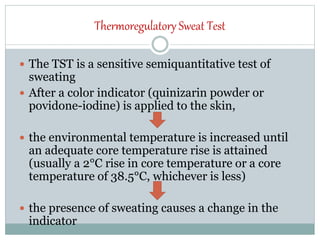
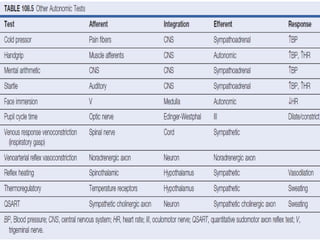
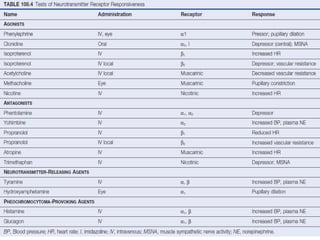
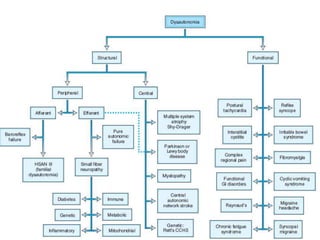
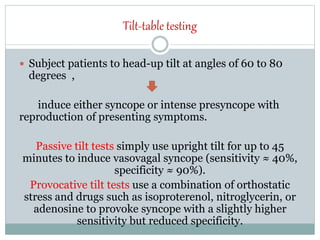
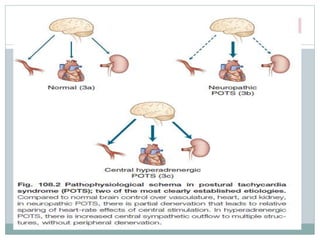
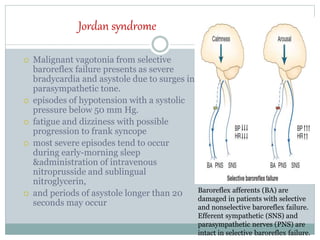
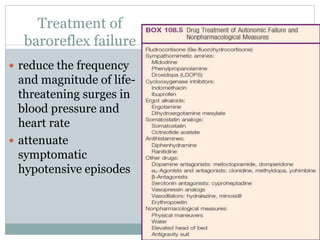
The document discusses the autonomic nervous system and its disorders. It begins by defining the autonomic nervous system and dividing it into the sympathetic and parasympathetic nervous systems. It then discusses methods of assessing autonomic function, including heart rate variation tests, Valsalva maneuver, quantitative sudomotor axon reflex test, and sympathetic skin response. Next, it covers autonomic disorders like reflex syncope, postural tachycardia syndrome, and functional gastrointestinal disorders. Finally, it discusses autonomic storms and Takotsubo cardiomyopathy, which result from excessive autonomic outflow.