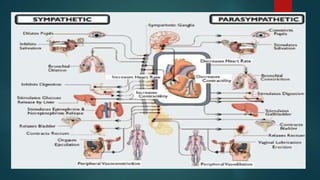
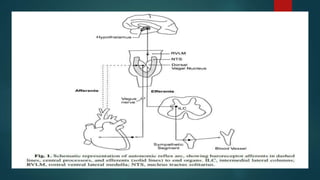
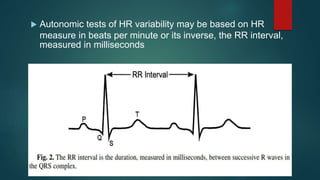
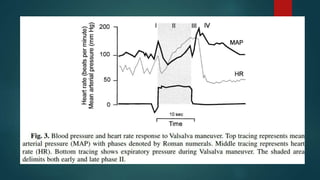
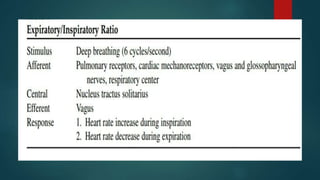
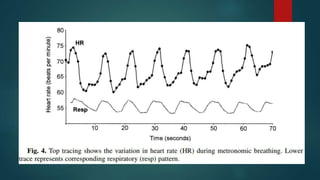
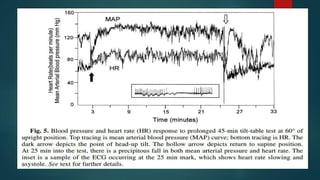
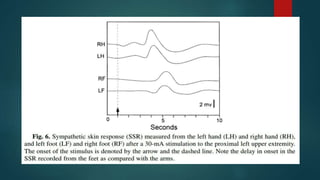
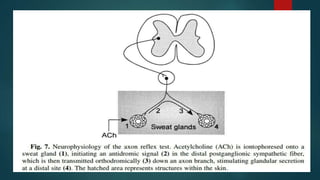
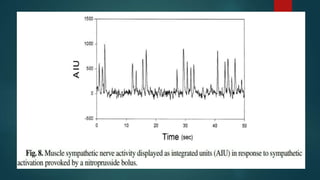
The document provides information on testing the autonomic nervous system. It discusses the challenges in testing the ANS as most structures are distant from the skin. It outlines some commonly used tests like heart rate response to the Valsalva maneuver which evaluates parasympathetic function by measuring heart rate changes during forced exhalation against closed glottis. The document also discusses indications for ANS testing and preparations patients need to make, like refraining from medications and heavy meals before tests.