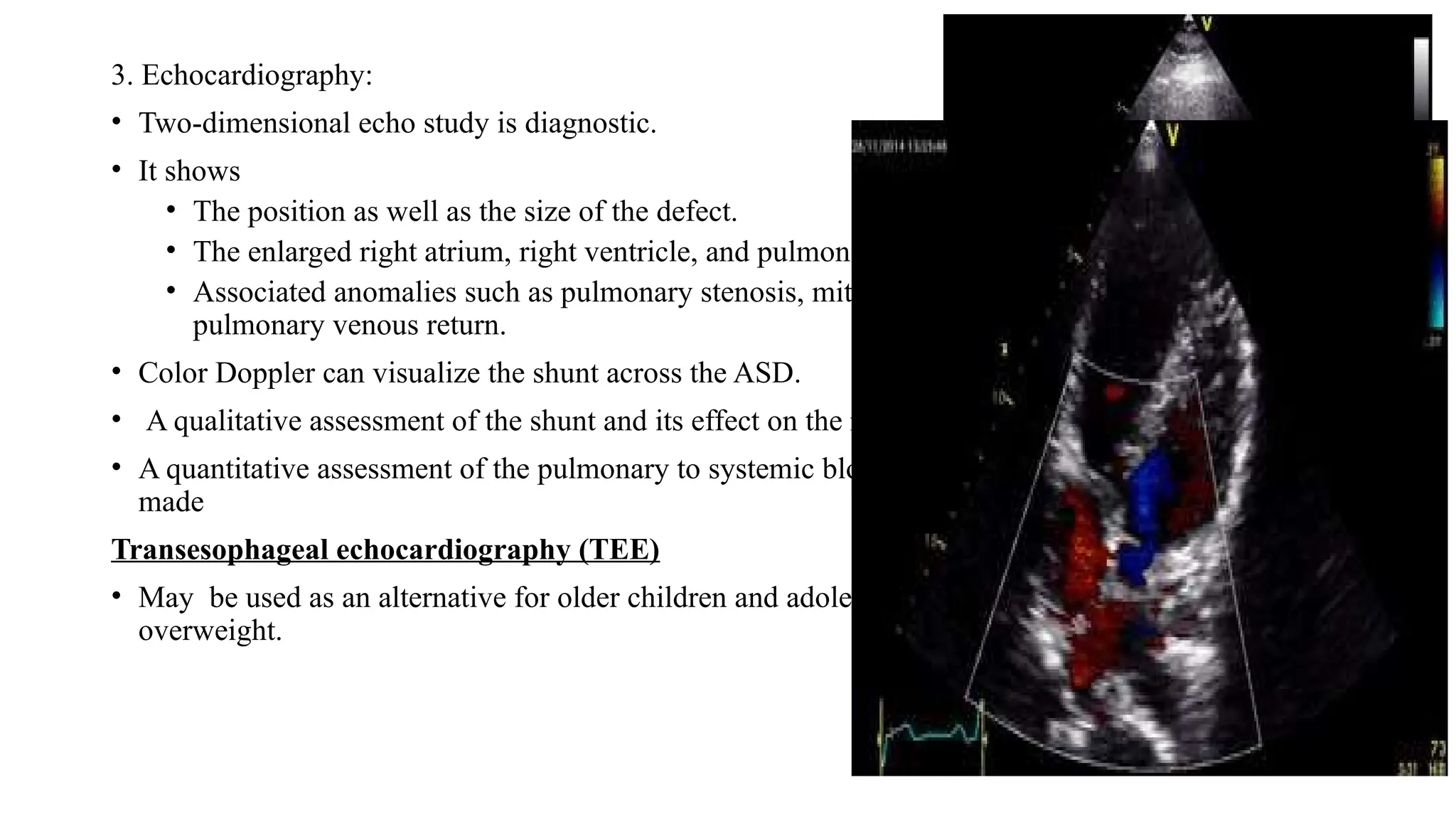
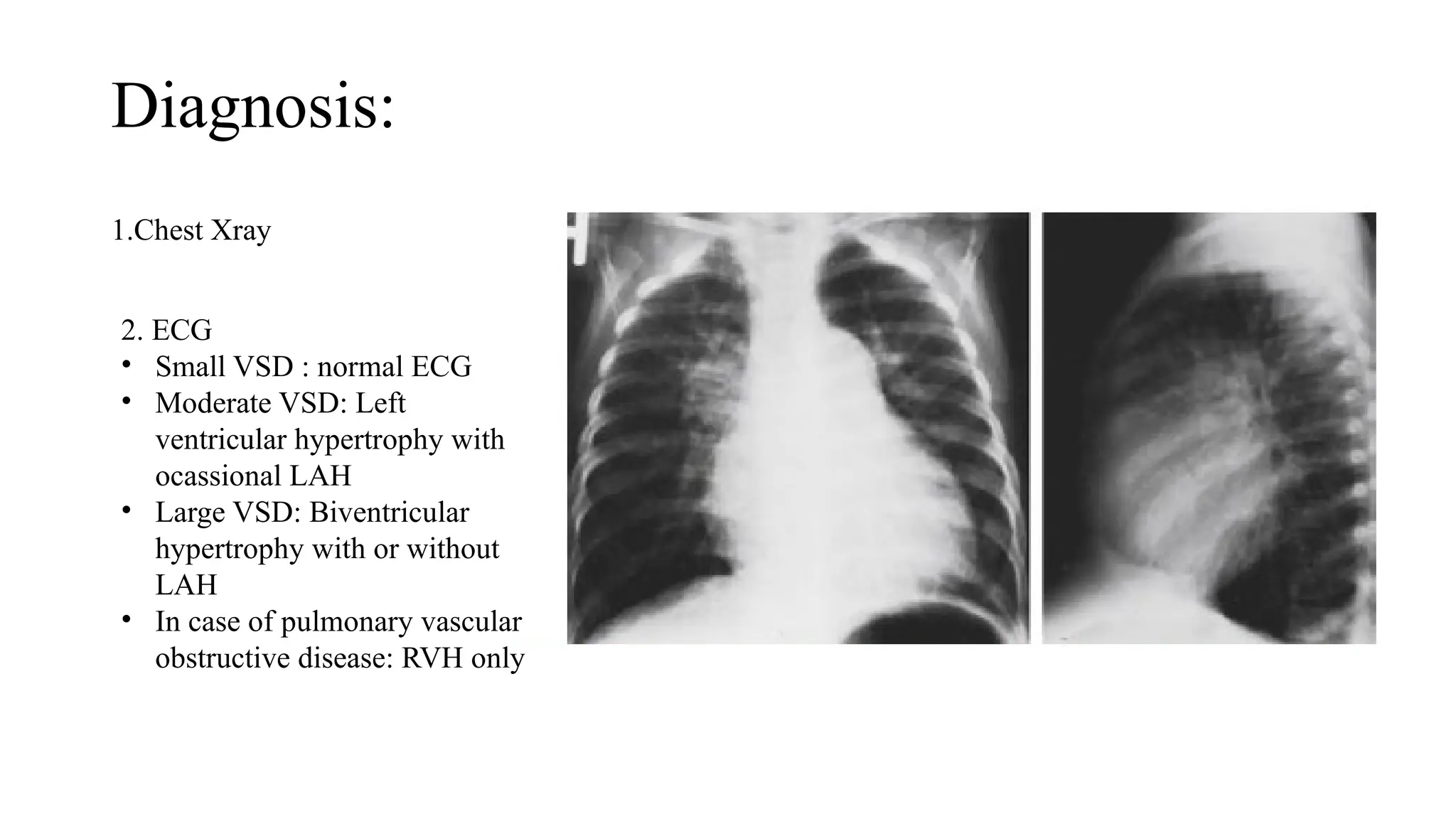
The document discusses acyanotic congenital heart diseases, specifically atrial septal defect (ASD) and ventricular septal defect (VSD), detailing their incidence, types, pathophysiology, clinical presentation, diagnosis, management, and complications. It highlights the prevalence of ASDs in children, the importance of echocardiography for diagnosis, and treatment options such as surgical and device closure for significant defects. VSD is also explored, including its classification, management strategies, and indications for surgical intervention, emphasizing the importance of timely treatment to prevent long-term complications.