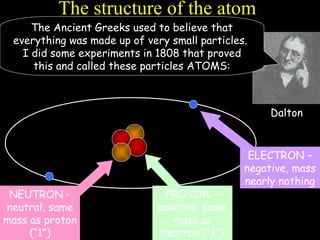
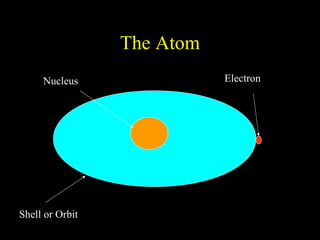
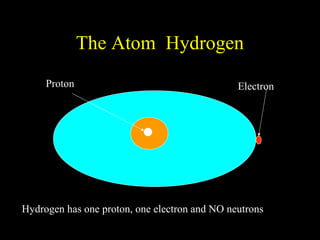
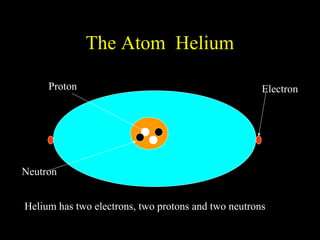
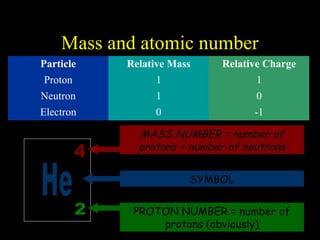
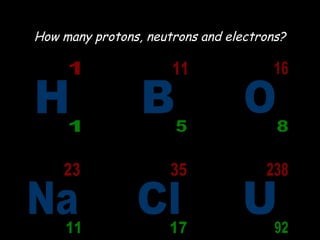
The document summarizes atomic structure. It explains that atoms are made up of protons, neutrons, and electrons. Protons are positively charged and have a mass of about 1 atomic mass unit. Neutrons are neutral and also have a mass of about 1 atomic mass unit. Electrons are negatively charged and have a negligible mass. The nucleus of an atom contains protons and neutrons, and electrons orbit the nucleus in shells. For example, hydrogen has 1 proton, 1 electron, and no neutrons, while helium has 2 protons, 2 neutrons, and 2 electrons. The mass number of an atom is the total number of protons and neutrons, and the proton number indicates how many protons an atom contains. Electron shells