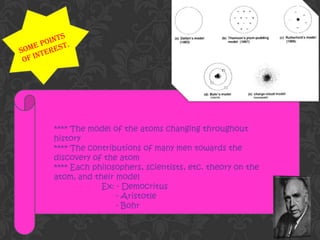
The document summarizes the models and contributions of several important scientists throughout history towards developing the modern understanding of atoms. It describes Democritus' early atomic theory of indivisible atoms in different shapes and sizes. John Dalton improved upon this with his billiard ball model of atoms and proposed atoms of elements are identical. J.J. Thomson's plum pudding model viewed atoms as positively charged material with electrons scattered about. Rutherford's gold foil experiment showed atoms have a small, dense nucleus. Niels Bohr incorporated this into his model of electrons orbiting the nucleus in defined energy levels. Werner Heisenberg introduced the concept of electron clouds rather than defined orbits. James Chadwick discovered the neutron in 1932,