This document discusses four major atomic models:
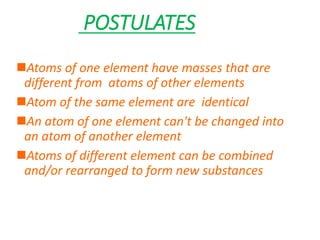
- Dalton's atomic theory from 1807 which proposed atoms of the same element are identical and atoms can combine to form new substances.
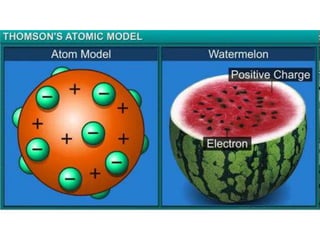
- Thomson's "plum pudding" model from 1904 which viewed the atom as a positively charged sphere with electrons embedded within it.
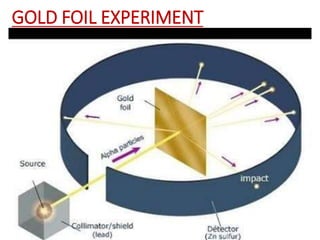
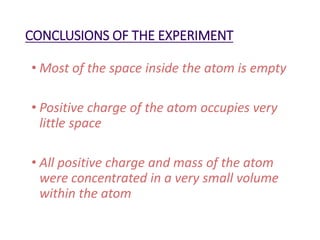
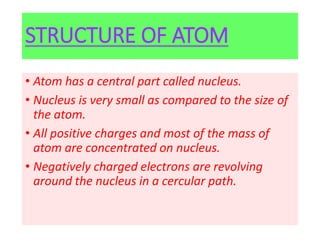
- Rutherford's 1909 "planetary model" based on his gold foil experiment, showing the atom consists of a small, dense nucleus surrounded by empty space with electrons orbiting the nucleus.
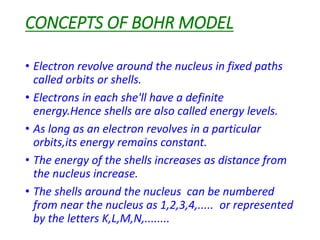
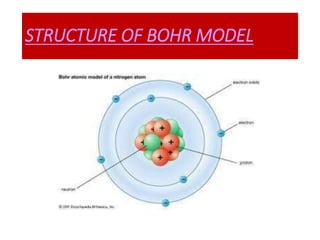
- Bohr's 1913 model which introduced the concept of distinct electron orbits or shells around the nucleus to explain atomic spectra. This model is closest to how atoms are currently understood.