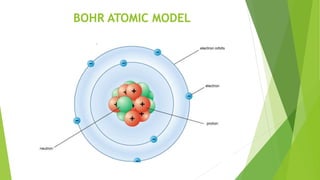
This document discusses atomic structure and different atomic models. It introduces atoms as the smallest particles that take part in chemical reactions, consisting of a nucleus surrounded by electrons. Several historic atomic models are mentioned, including J.J. Thomson's, Rutherford's, and Bohr's models. Bohr's model from 1913 proposed that electrons orbit the nucleus in fixed shells and energy is released as photons when electrons change shells. The document concludes with important postulates of Bohr's model, which helped explain atomic structure with a nucleus and orbiting electrons.