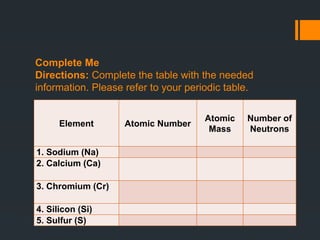
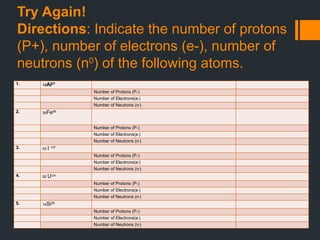
The document explores atomic structure, focusing on protons, neutrons, and electrons, and how they relate to atoms and elements in the periodic table. It includes educational goals for learners to identify and understand these atomic components and their significance in matter. Additionally, it provides activities and guide questions to reinforce students' comprehension of atomic numbers, mass numbers, and chemical changes.