Embed presentation
Download to read offline


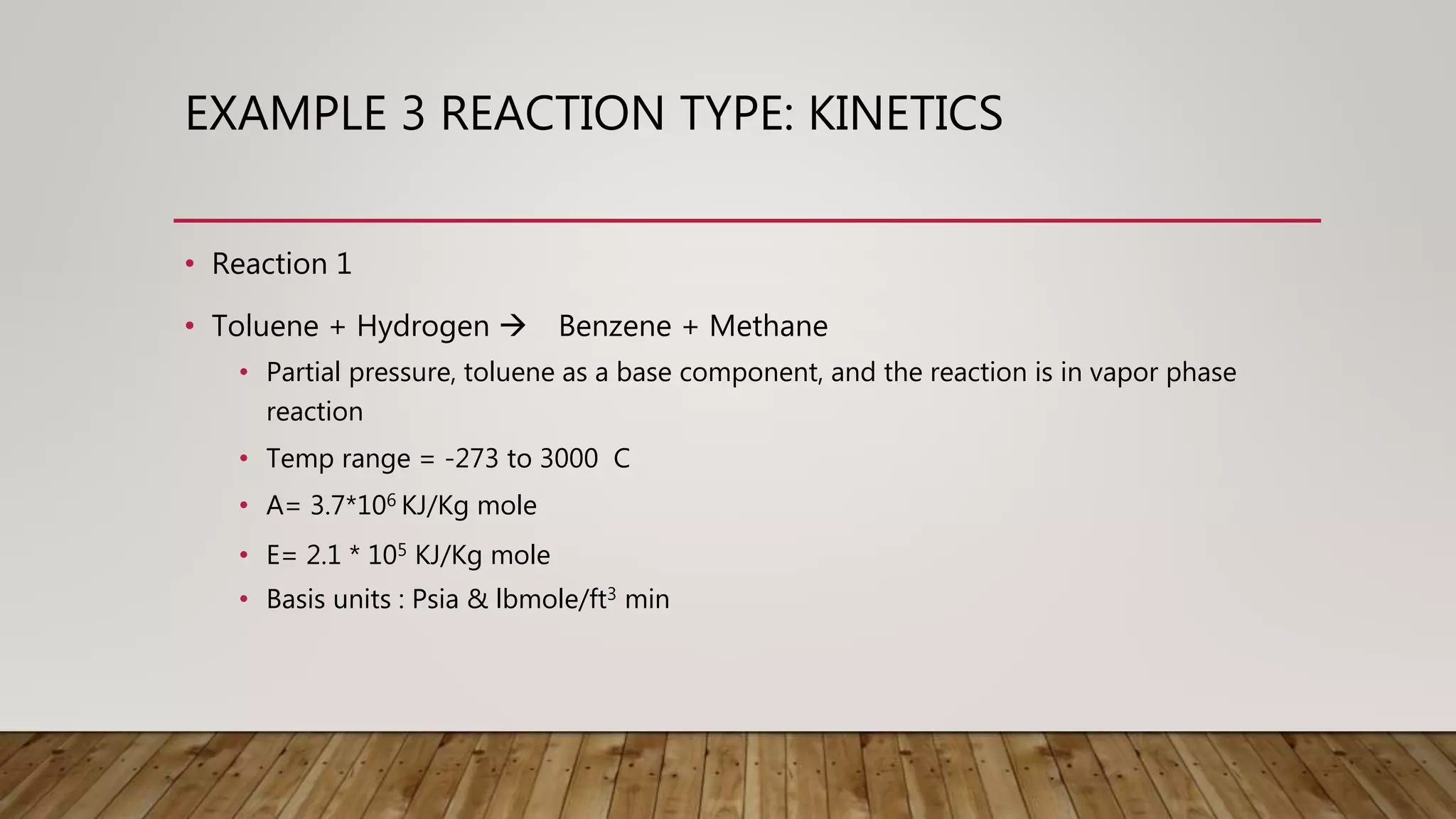

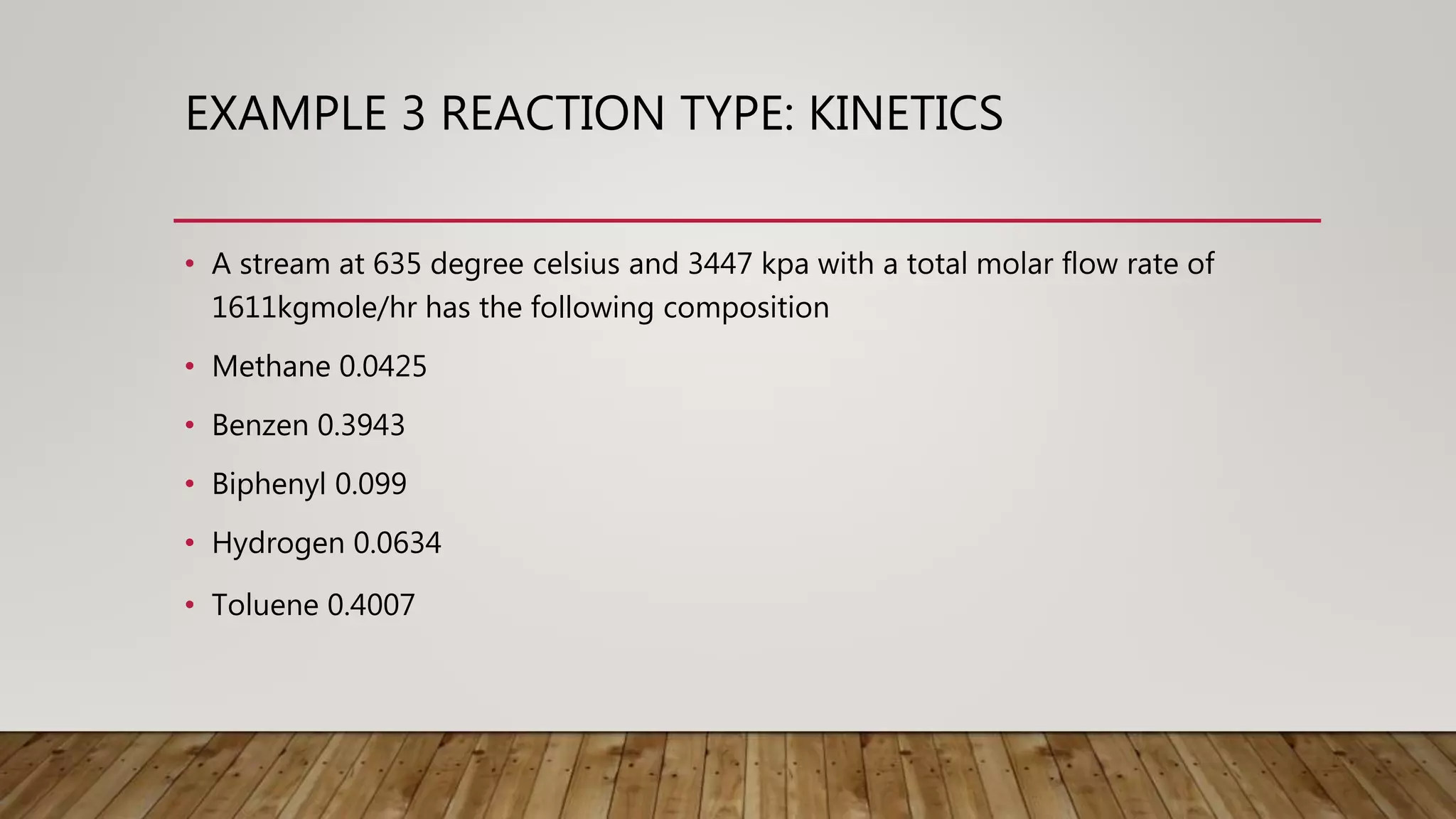


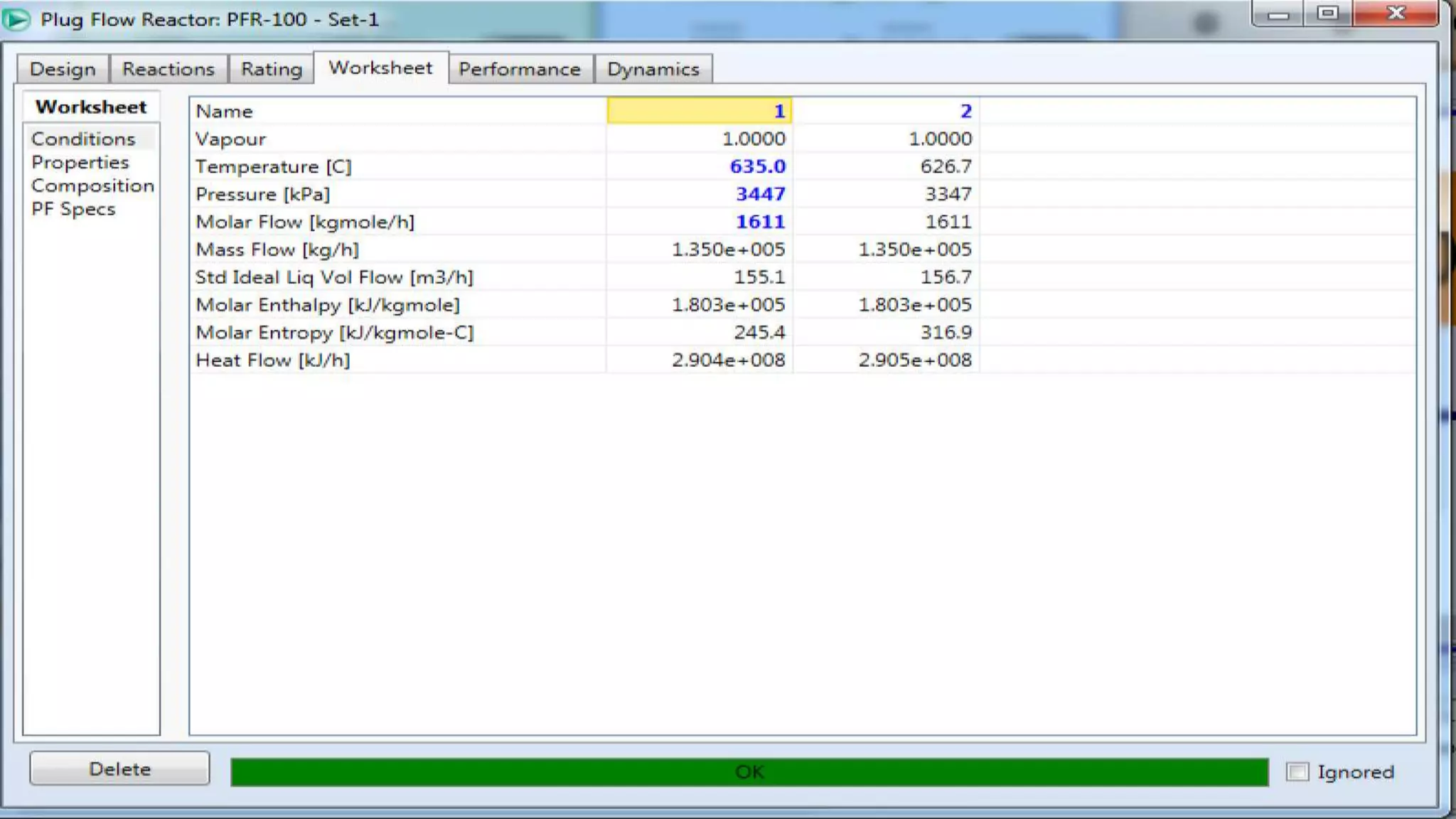

This document provides examples of chemical reaction simulations. Example 2 simulates an equilibrium reactor where CO and H2O react to form CO2 and H2. The vapor from a conversion reactor is sent to this equilibrium reactor along with steam. Example 3 simulates two kinetic reactions in a plug flow reactor: 1) Toluene + Hydrogen reacting to form Benzene + Methane, and 2) two Benzene molecules reacting to form Biphenyl + Hydrogen. A stream with various components enters the plug flow reactor at high temperature and pressure. The reactor has specified dimensions and pressure drops are noted. The stream is then cooled before being sent to a separator.








