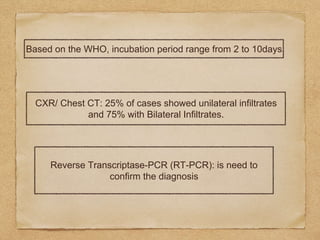
The document reviews the 2019-nCoV outbreak, first identified in Wuhan, China, and addresses its transmission, clinical manifestations, and diagnosis. It discusses the significant risk of human-to-human transmission confirmed in various countries, the symptoms of patients, and the importance of early detection and prevention measures. It also highlights the case fatality rate and the need for quarantine and hygiene practices to manage the outbreak.