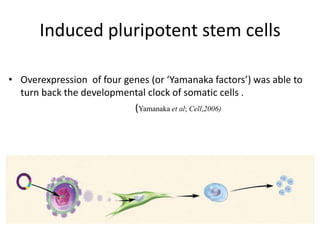
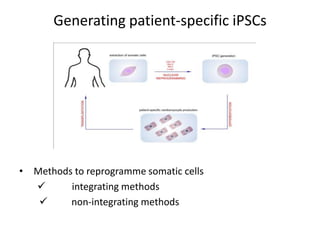
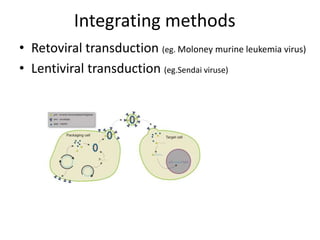
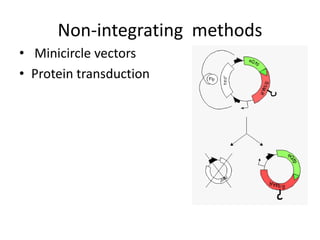
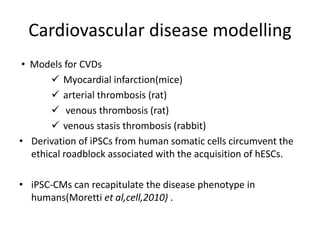
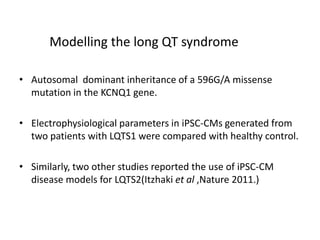
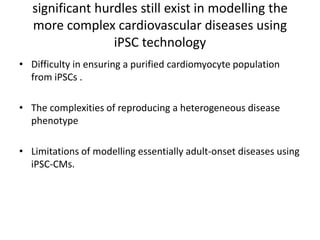
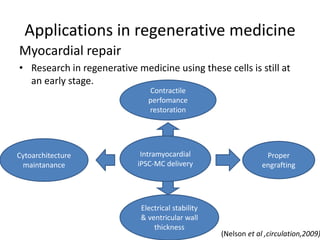
Cardiovascular diseases are the leading cause of death globally, with 17.3 million deaths in 2008. Induced pluripotent stem cells (iPSCs) offer potential treatments by generating patient-specific heart cells. iPSCs are generated through reprogramming somatic cells using genes like Yamanaka factors. Models of diseases like long QT syndrome using iPSC-derived cardiomyocytes have helped test drugs and uncover disease mechanisms. However, challenges remain in ensuring pure cardiomyocyte populations and modeling complex diseases. If addressed, iPSCs may help regenerative medicine efforts through cell transplantation and screening new drugs for cardiac toxicity.