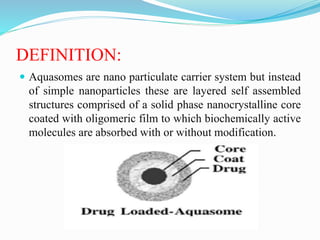
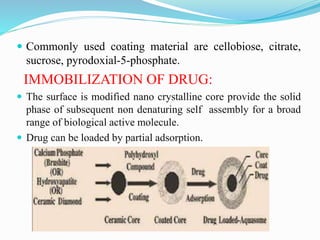
Aquasomes are nano particulate carrier systems comprised of a solid crystalline core coated with an oligomeric film for absorbing active molecules. They are spherical structures between 60-300nm that use self-assembly of macromolecules. The core is prepared using materials like tin oxide or calcium phosphate then coated with carbohydrates through lyophilization. Drugs can then be loaded through adsorption. Applications include delivery of vaccines, enzymes, genes, hemoglobin, and insulin where the aquasome protects fragile molecules and targets delivery.