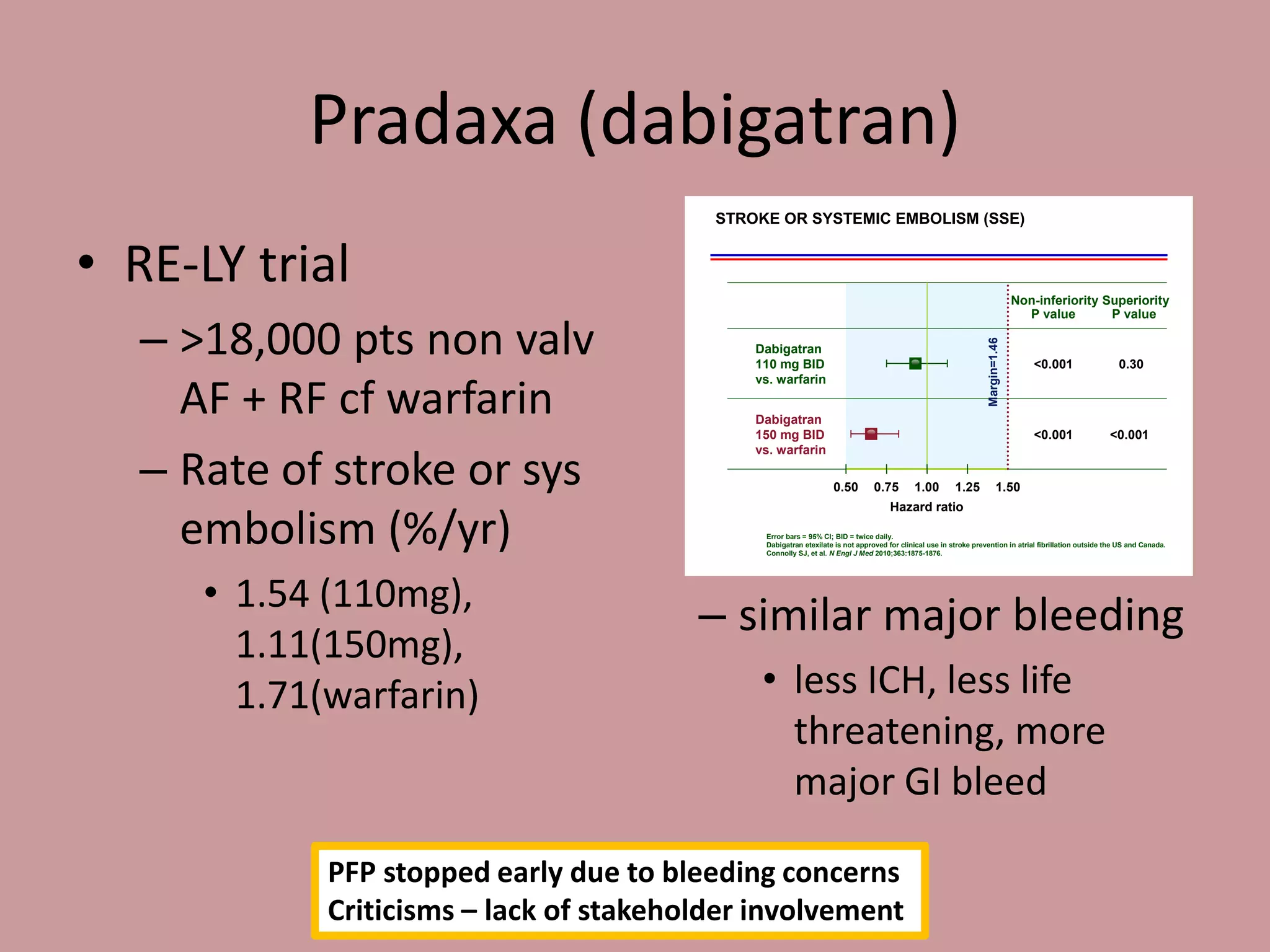
The document discusses two newer anticoagulants, Pradaxa (dabigatran) and Xarelto (rivaroxaban). It summarizes their mechanisms of action, indications, dosing, and monitoring. It notes that neither drug has a proven reversal agent. The summary emphasizes that these drugs are useful for certain patients but have no simple test to measure their effect. It advises that management of bleeding involves resuscitation, treating the source, stopping the drug, and contacting a hematologist, as the drugs cannot be readily reversed due to their short half-lives.