This document discusses NOAC anticoagulants and their reversal agents. It provides information on:
1. The advantages of NOACs over VKAs including their predictability, fewer drug interactions, and improved safety profile.
2. A meta-analysis found NOACs were associated with lower risks of major bleeding, fatal bleeding, and intracranial bleeding compared to VKAs.
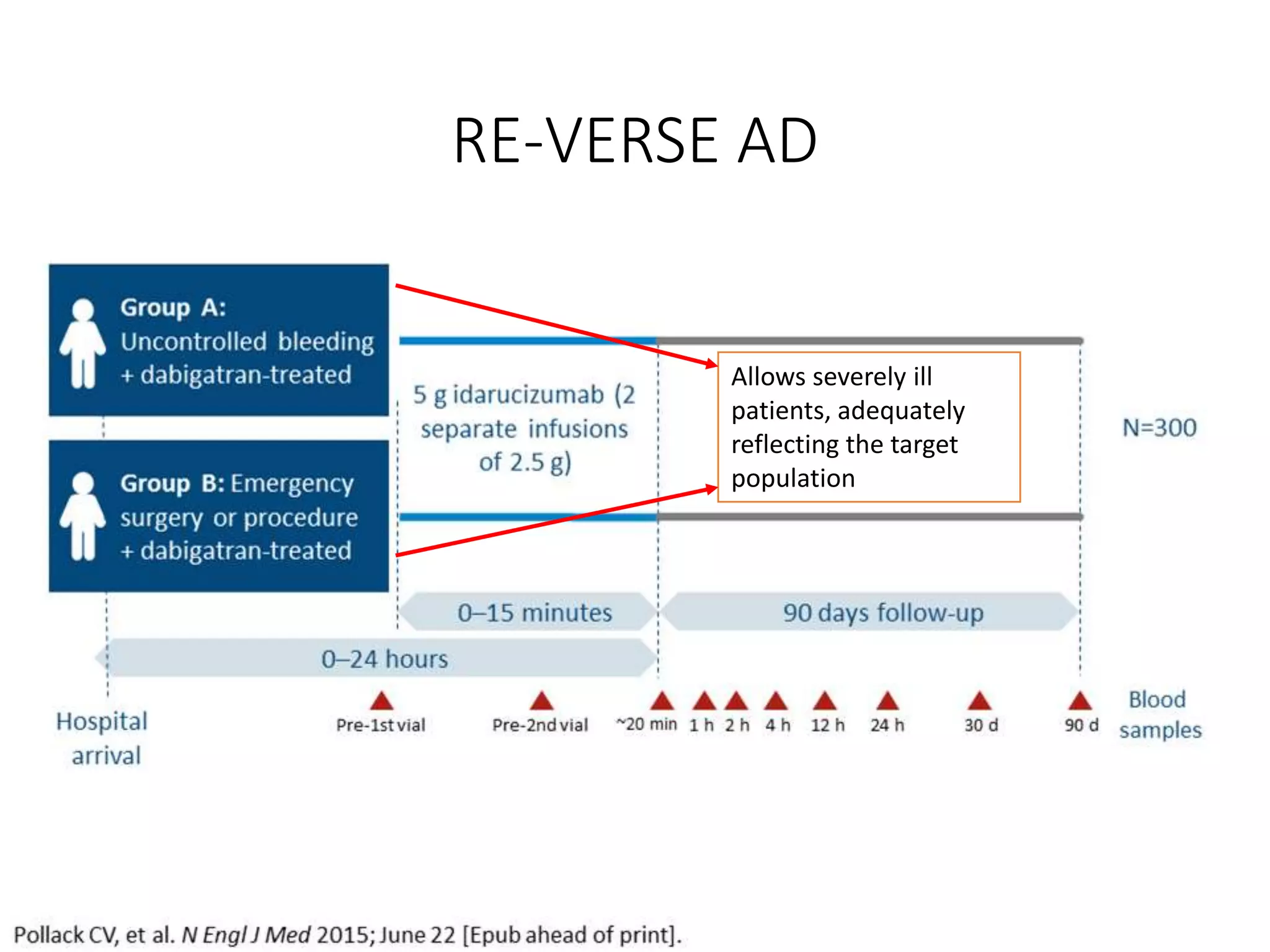
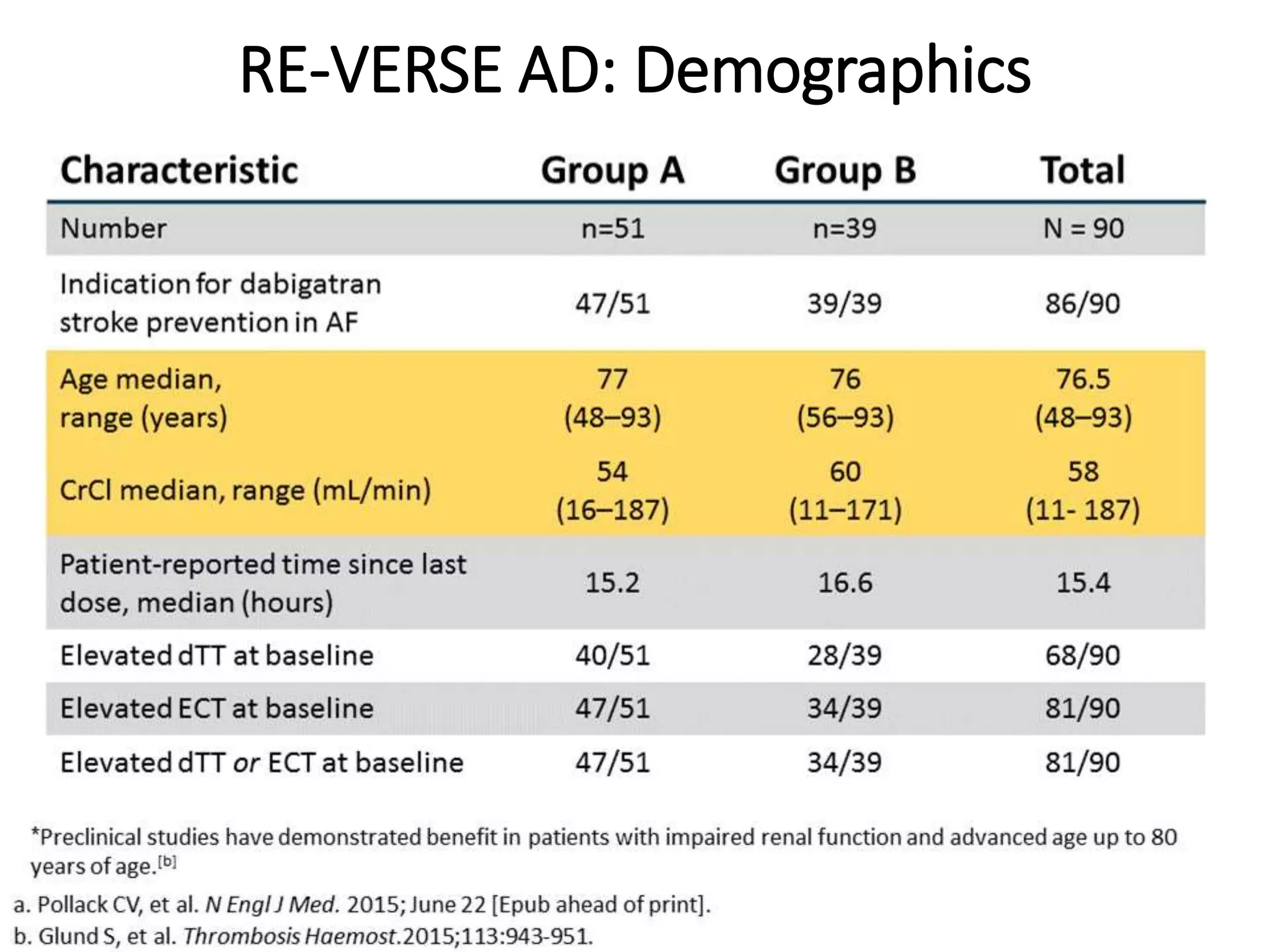
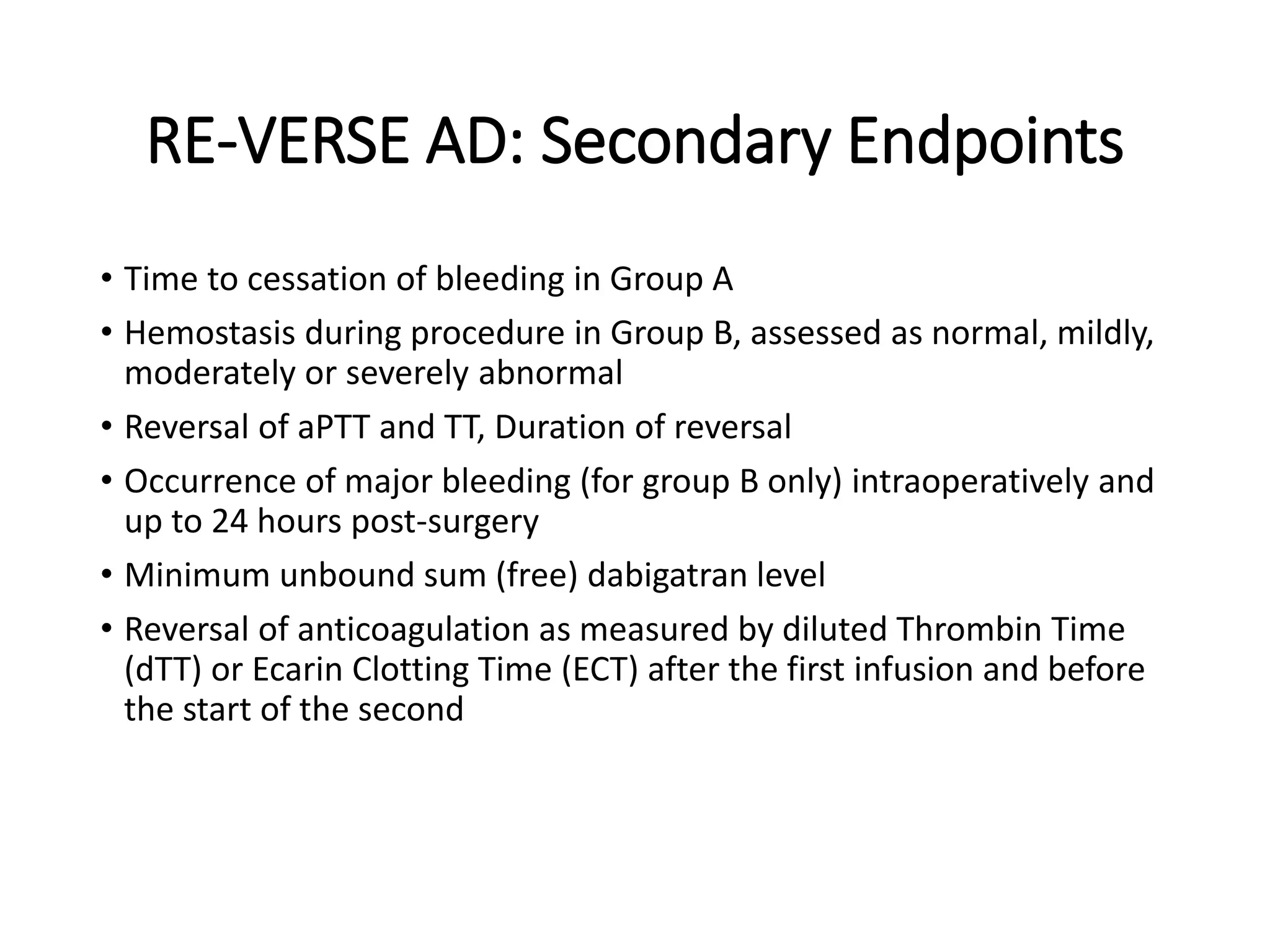
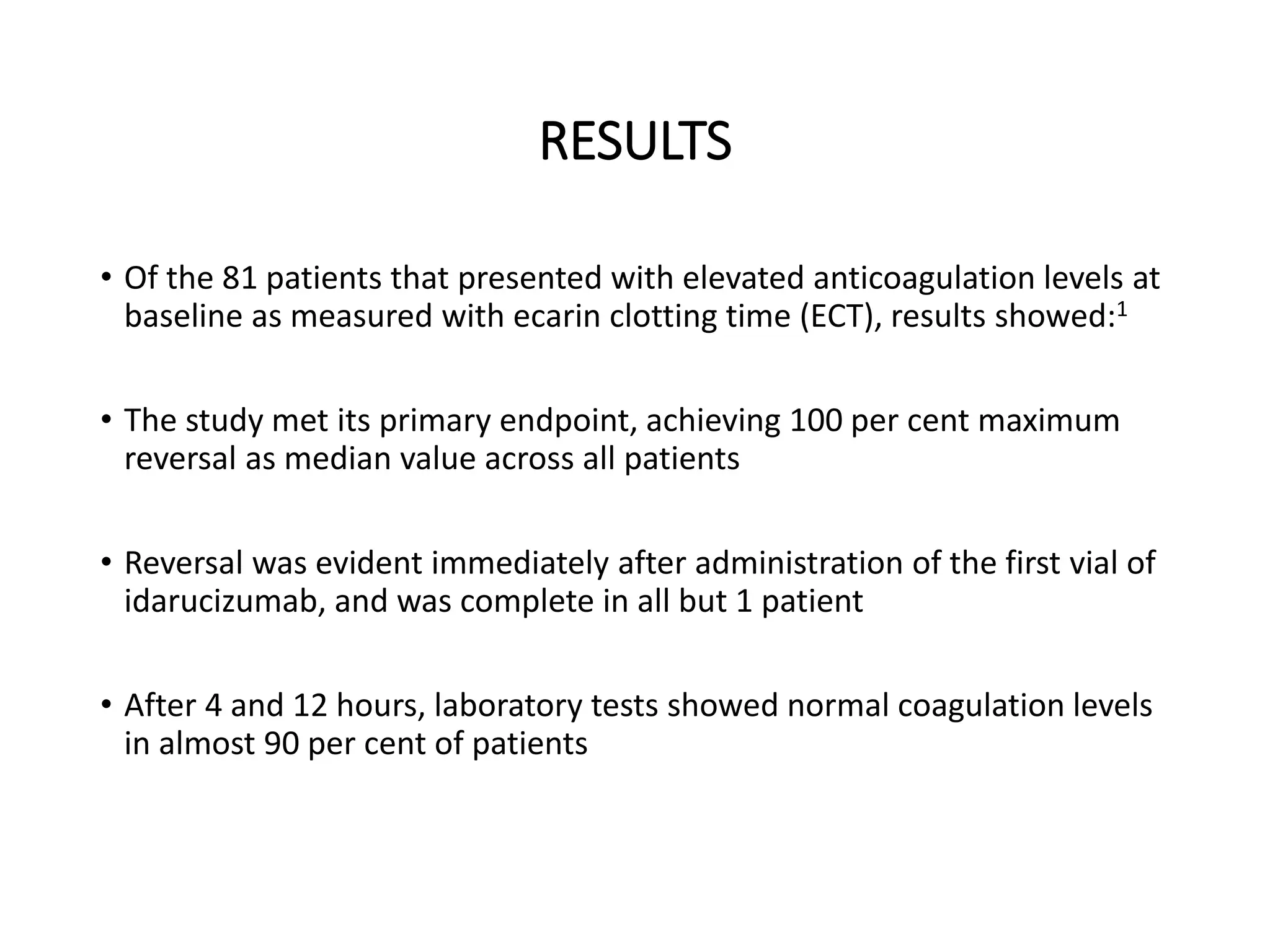
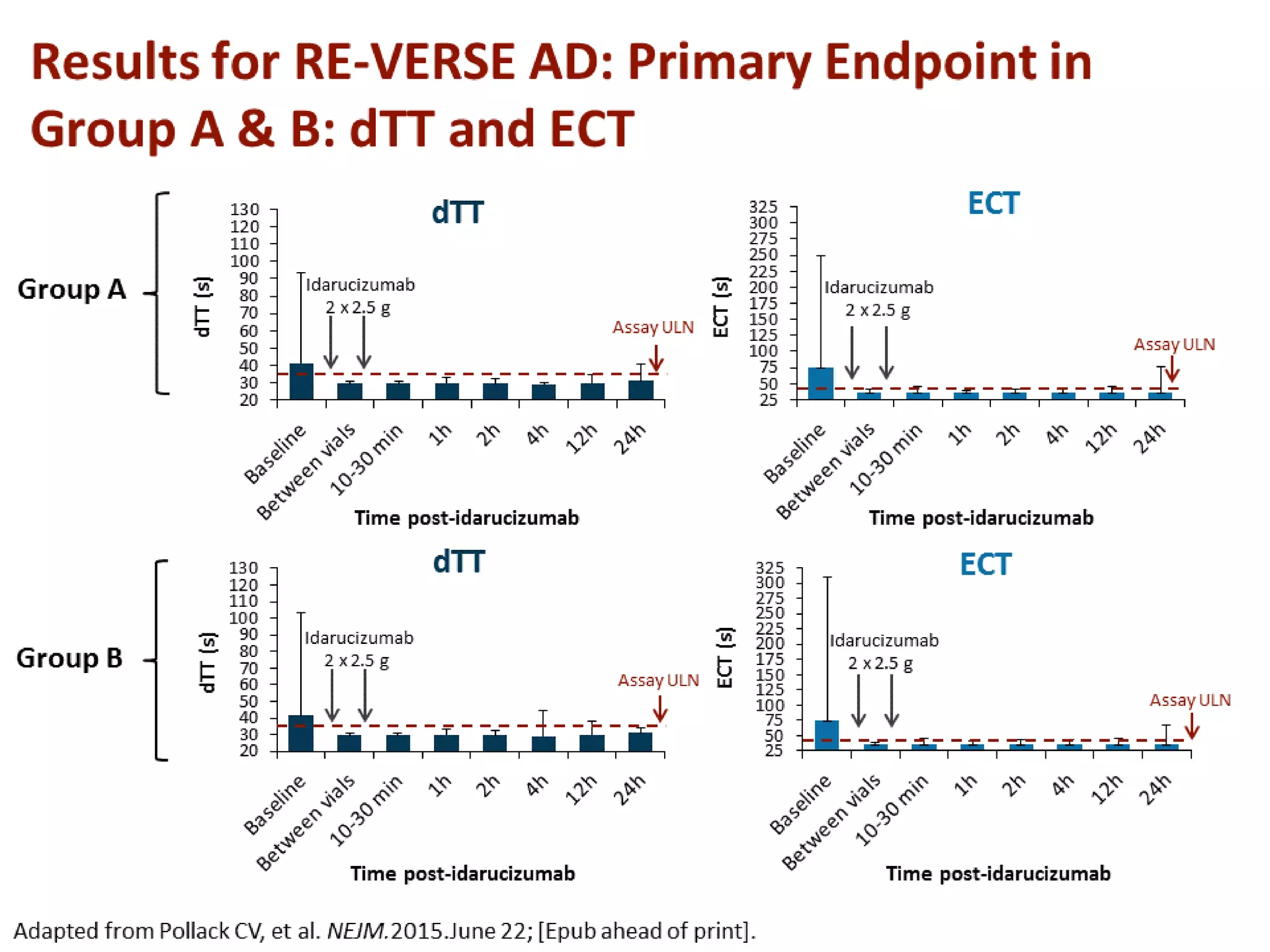
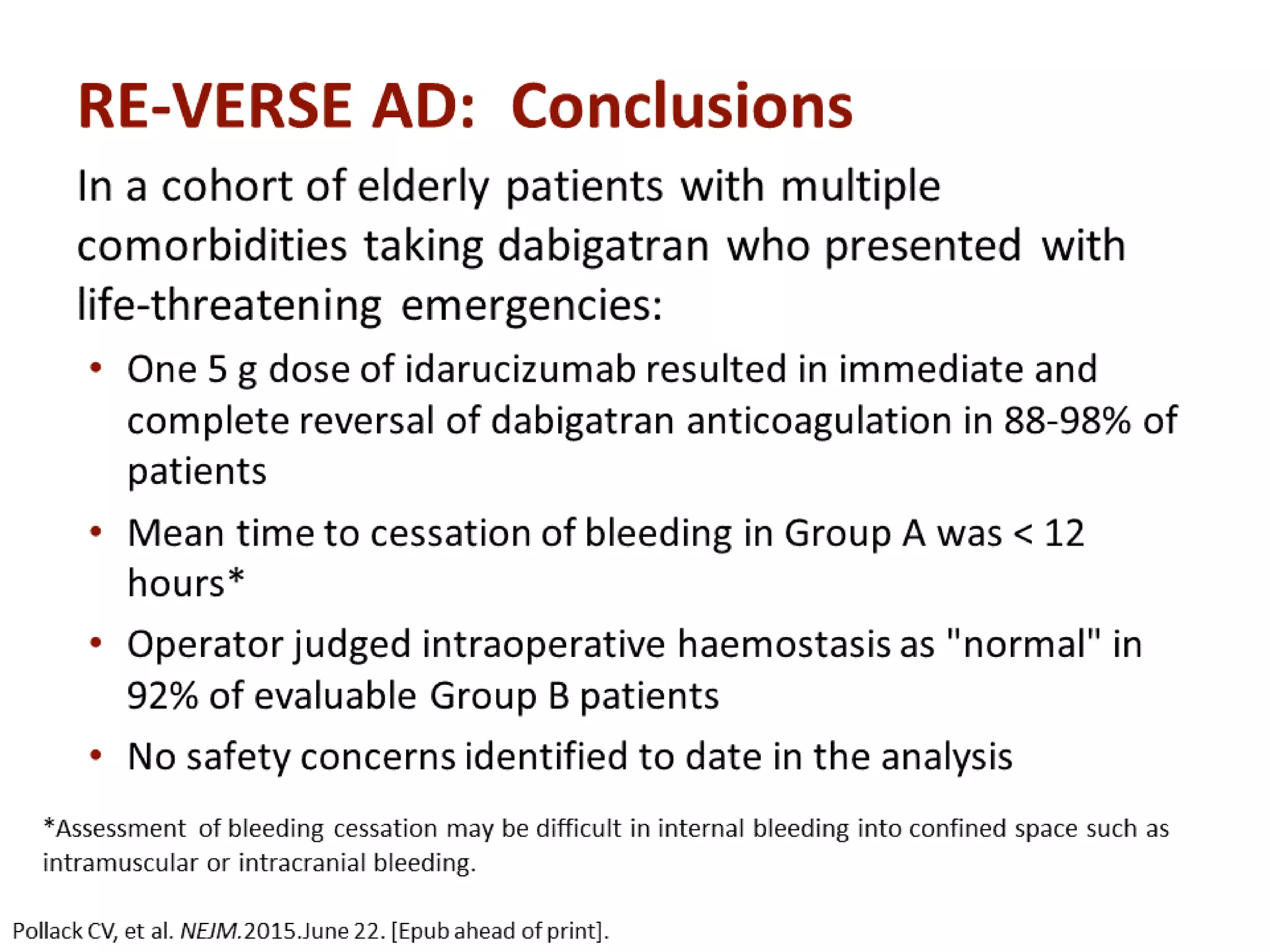
3. Idarucizumab, a specific reversal agent for dabigatran, demonstrated 100% reversal of dabigatran's anticoagulant effect based on interim results from the RE-VERSE AD trial of 90 patients with uncontrolled bleeding or those requiring emergency surgery.


![BLEEDING RISK
• Risks of bleeding from NOACs are generally lower or similar to other
agents, with some limited exceptions.
• The overall risk of bleeding with NOACs versus vitamin K antagonists was
reviewed in a meta-analysis of 12 randomized trials that included
102,607 patients with atrial fibrillation or venous thromboembolism.
• Compared with vitamin K antagonists, NOACs were associated with
lower risks of
• major bleeding (relative risk [RR] 0.72; 95% CI 0.62-0.85),
• fatal bleeding (RR 0.53; 95% CI 0.43-0.64), and
• intracranial bleeding (0.43; 95% CI 0.370.50);
• Major gastrointestinal bleeding was not increased (RR 0.94; 95% CI 0.75-
1.99)](https://image.slidesharecdn.com/antidotefornoacs-161103130856/75/Antidote-for-NOACs-3-2048.jpg)