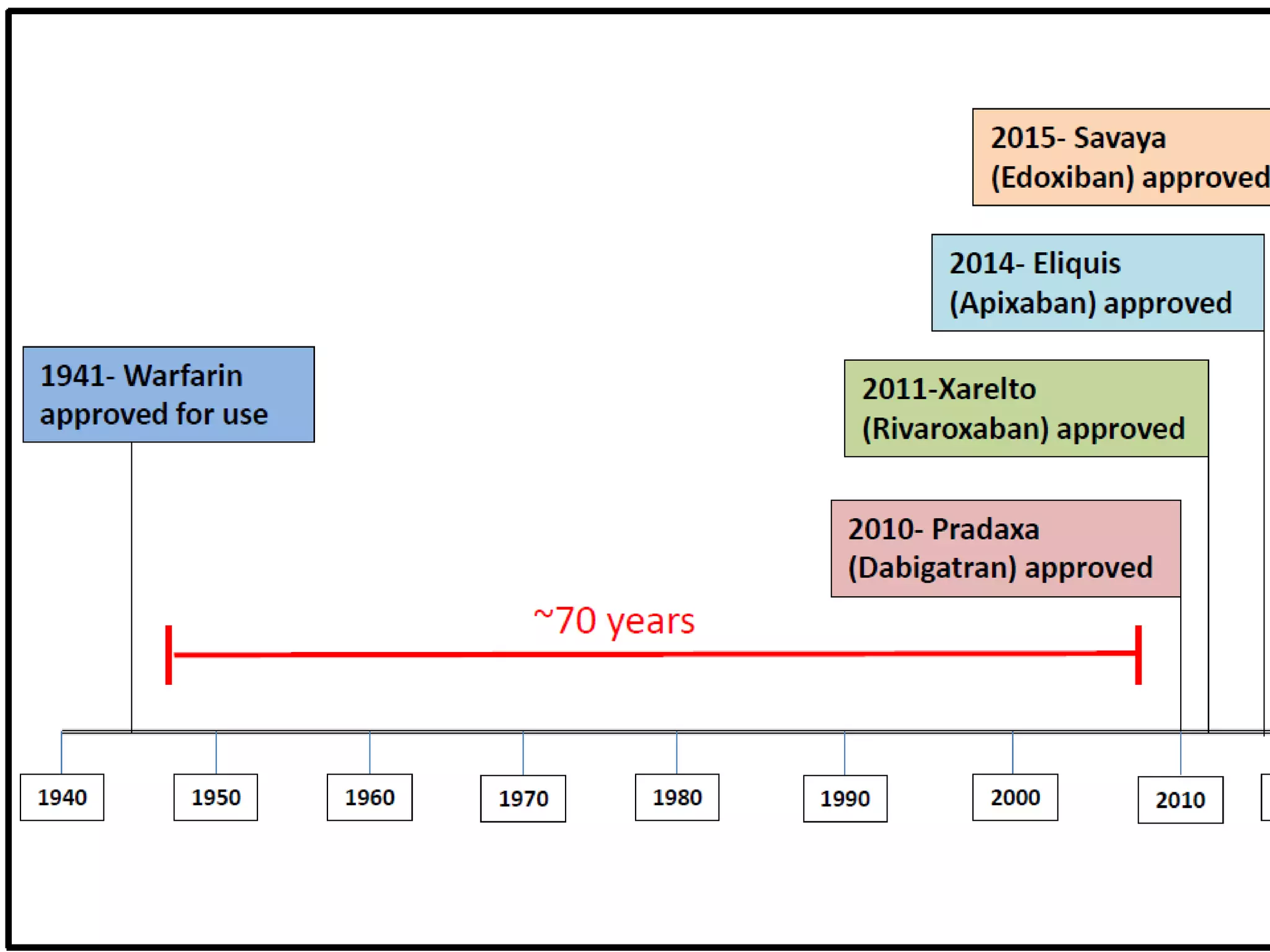
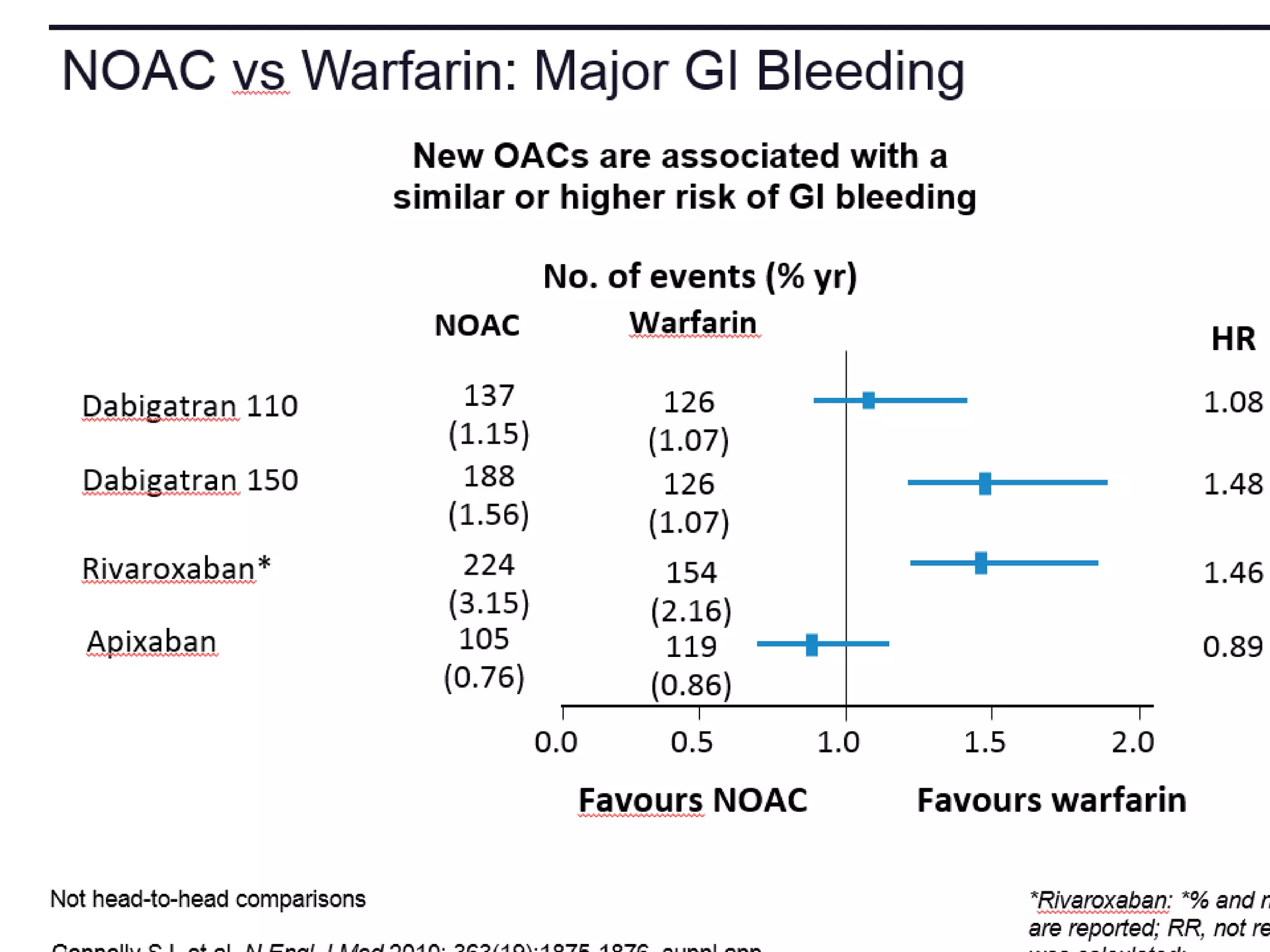
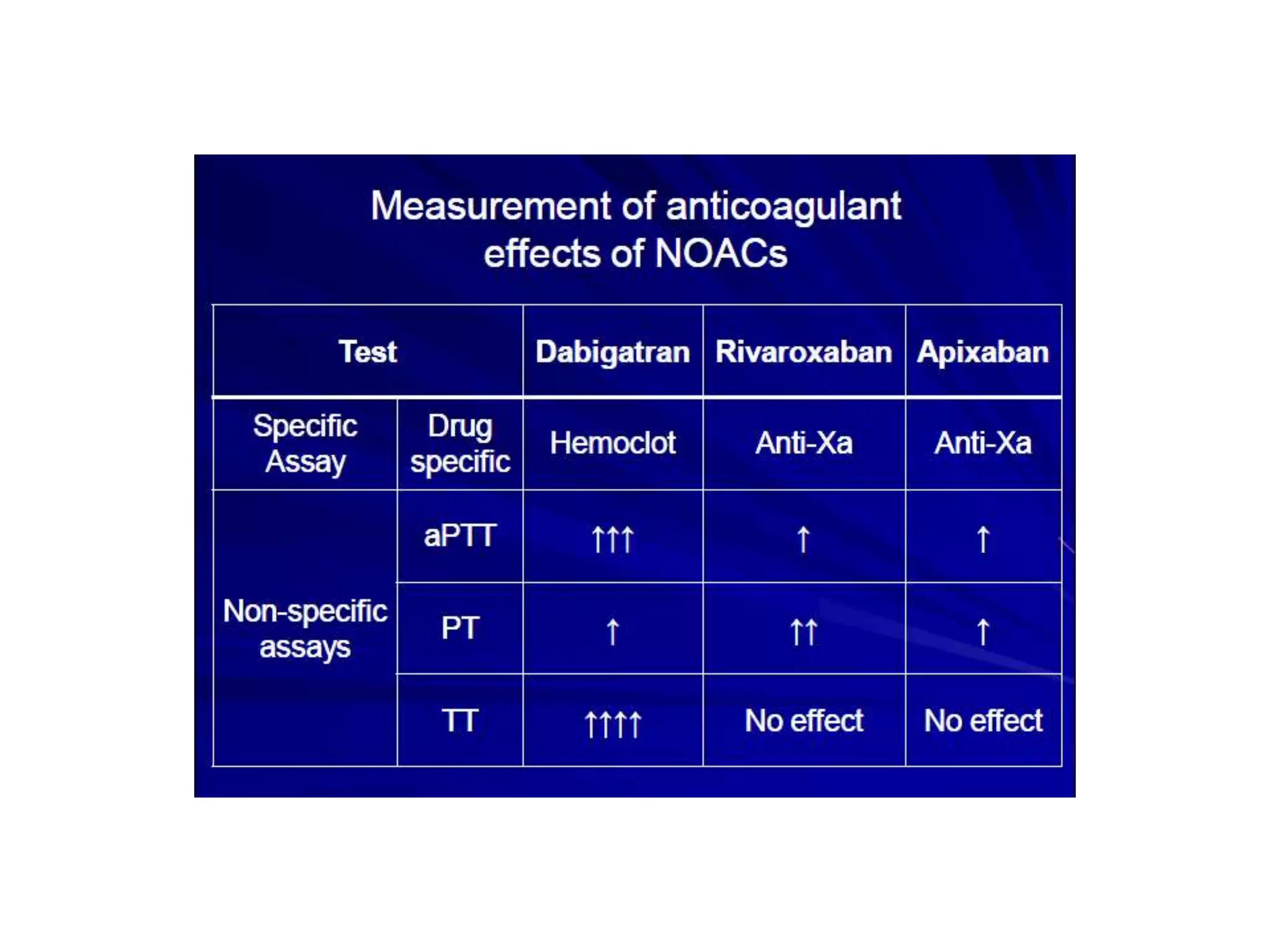
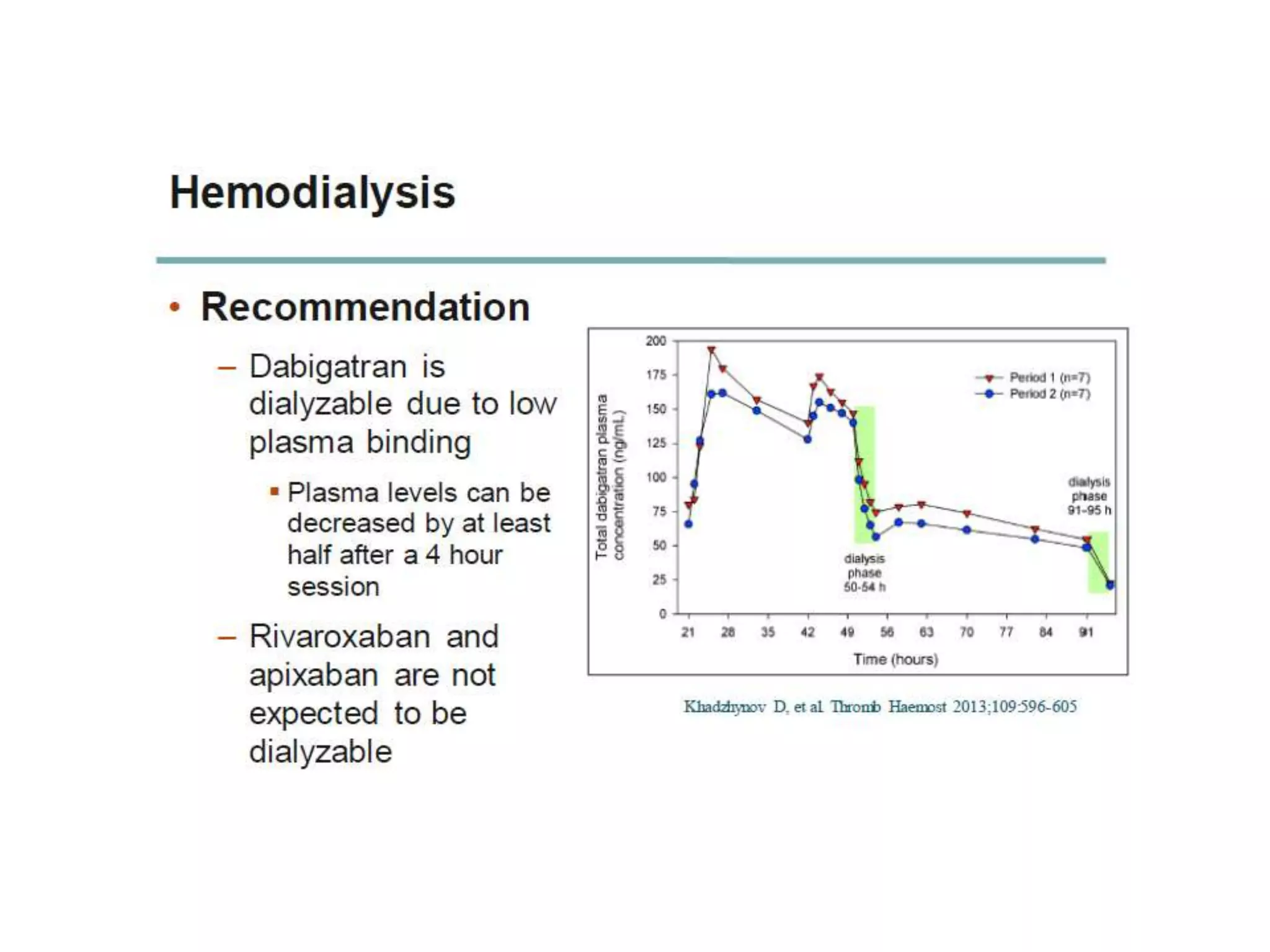
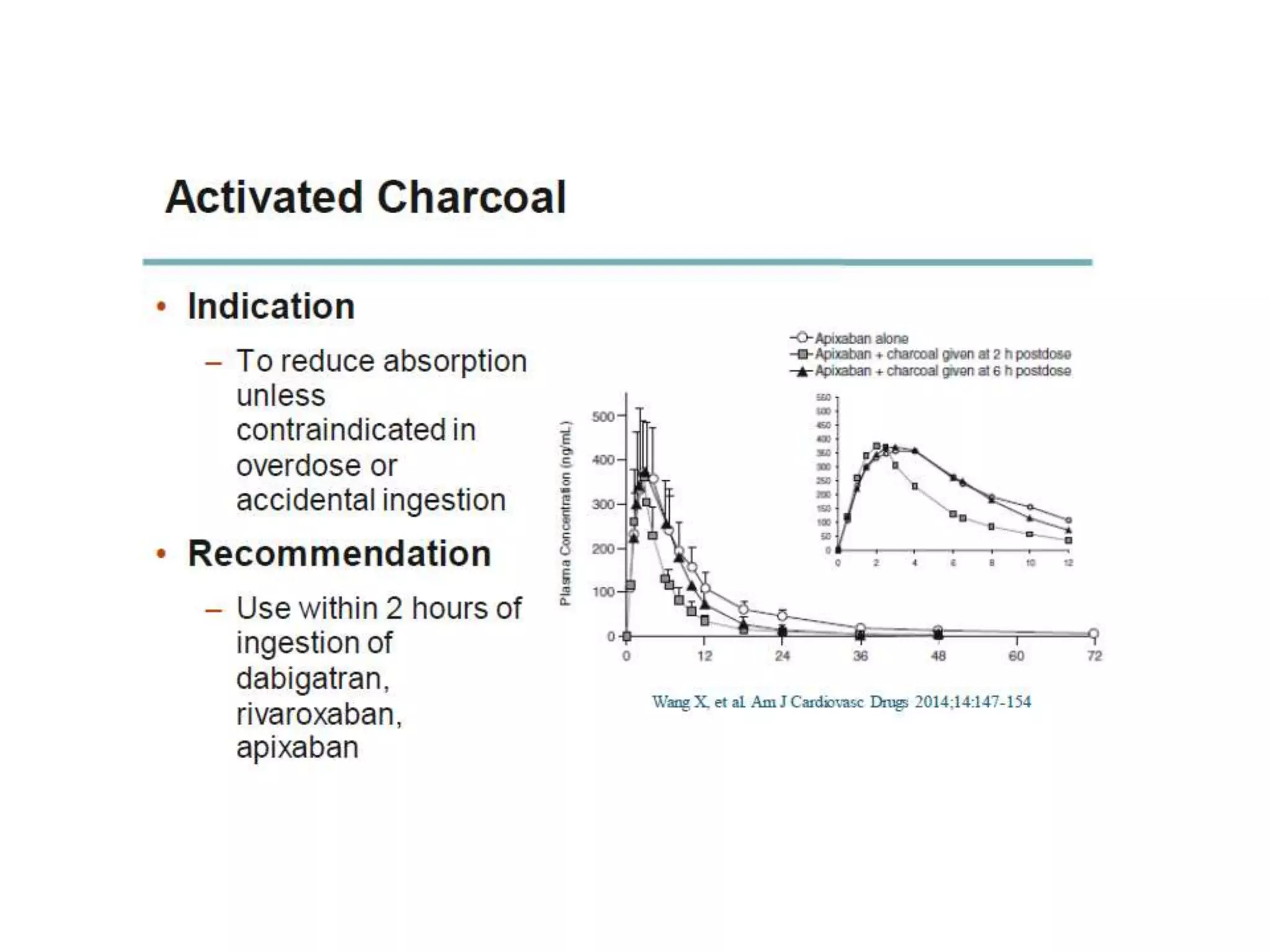
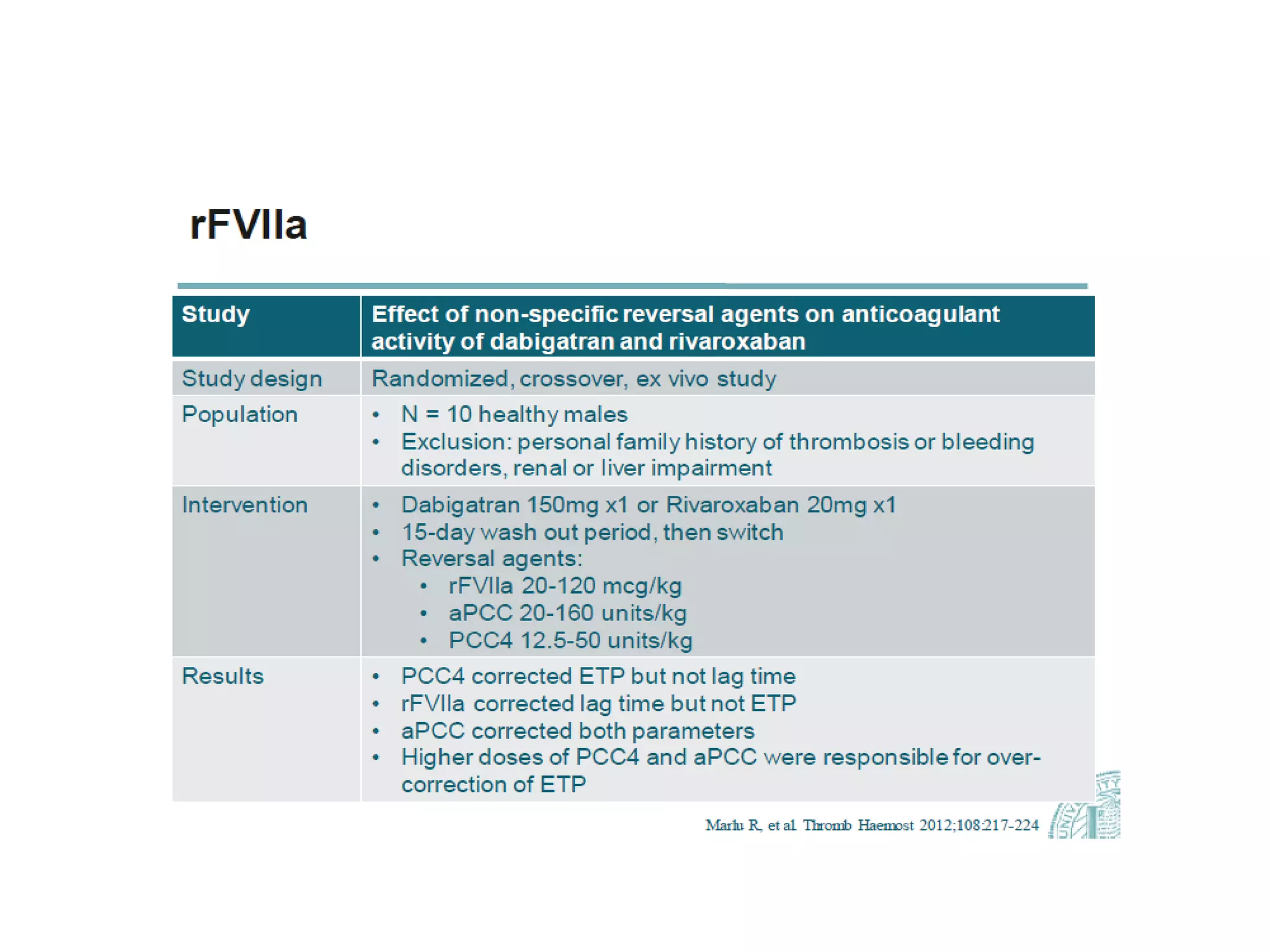
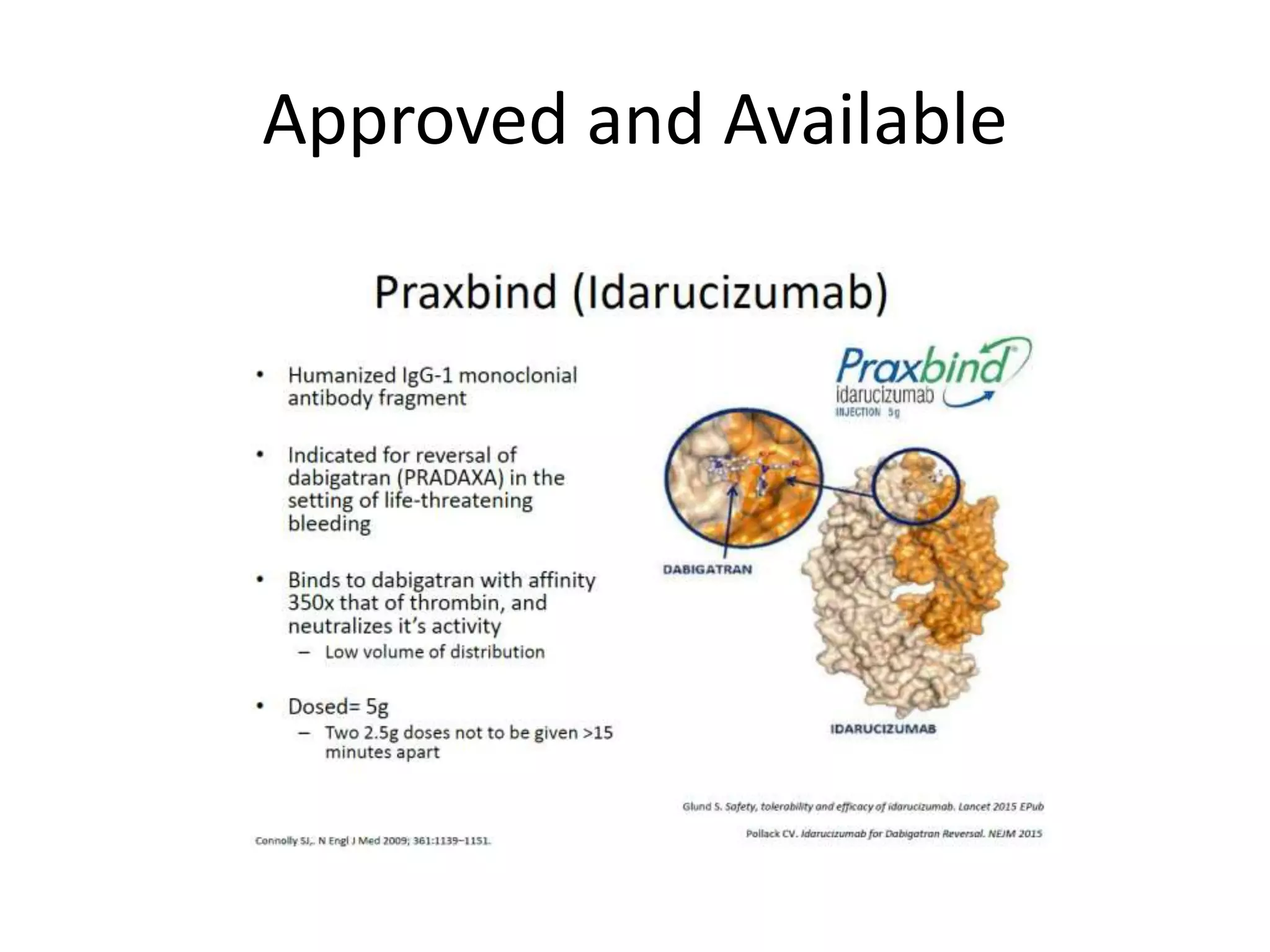
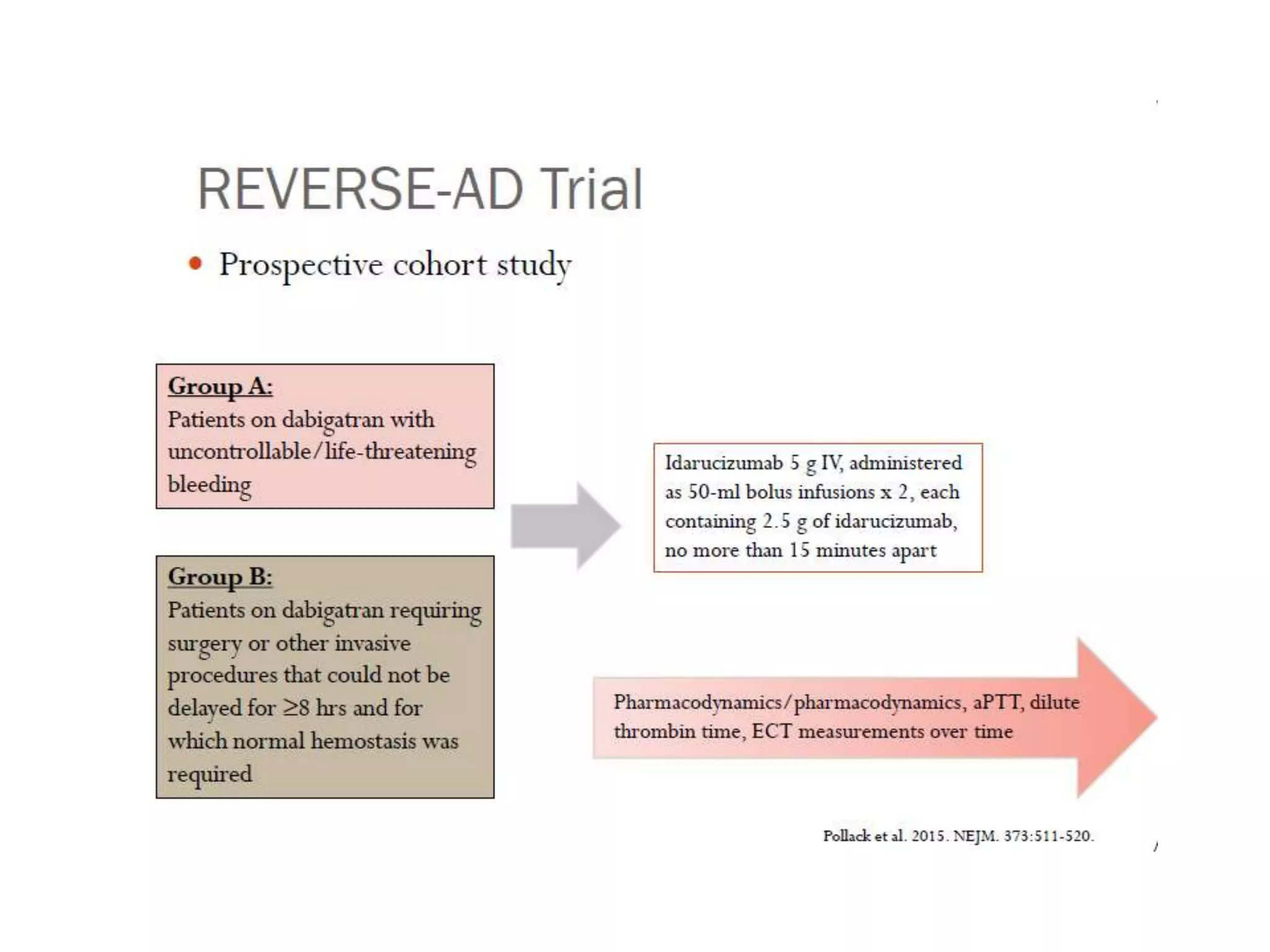
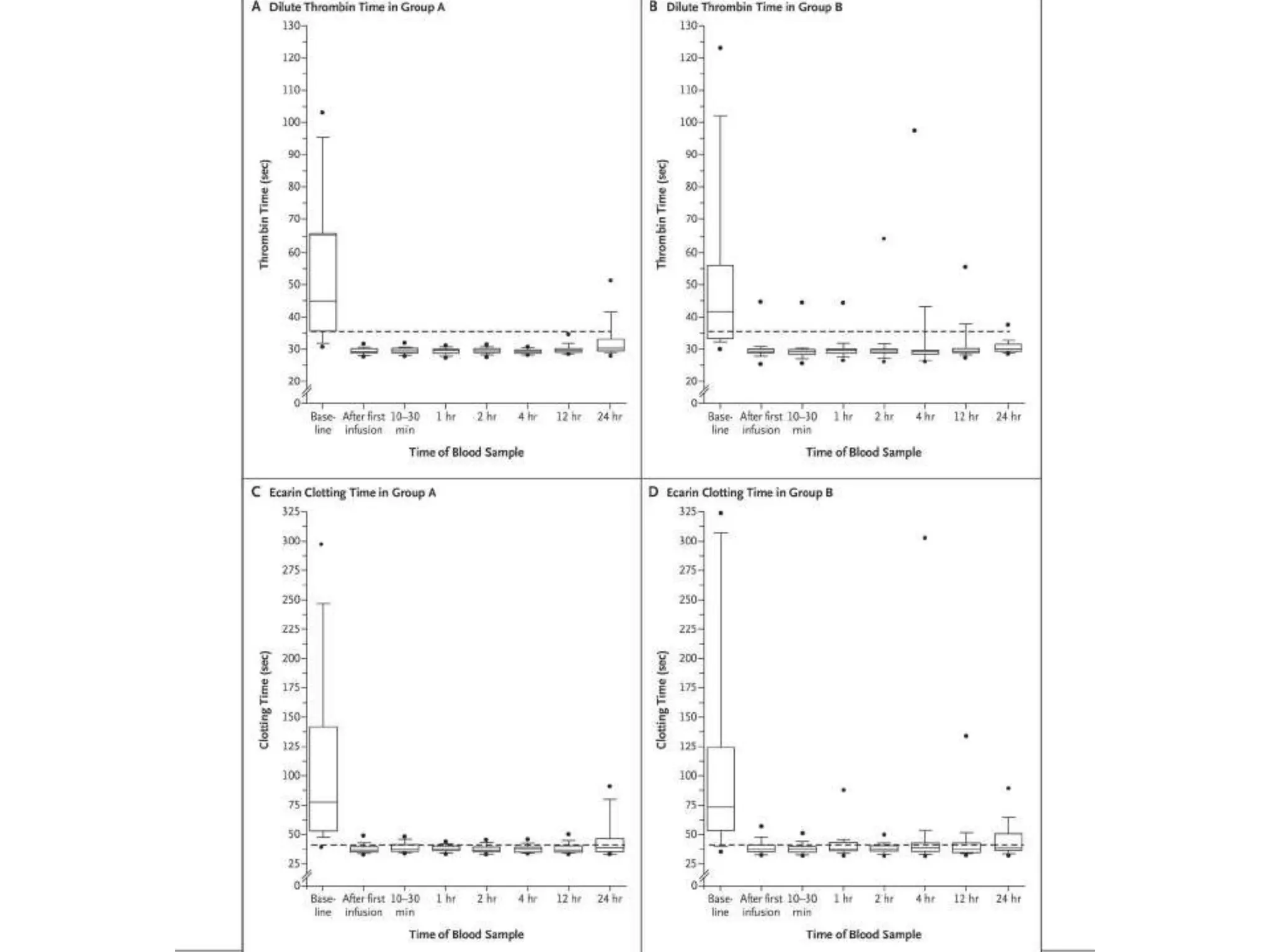
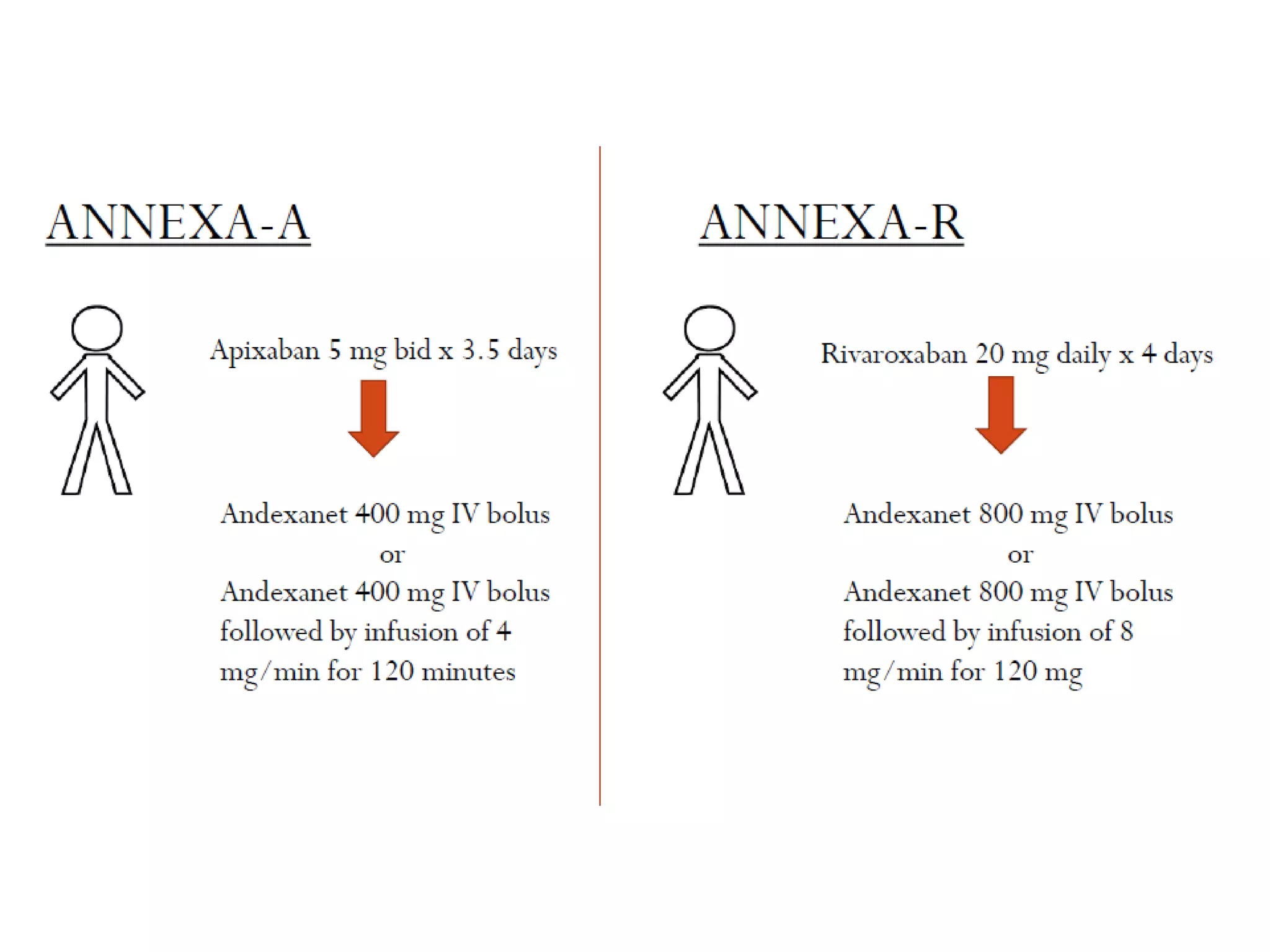
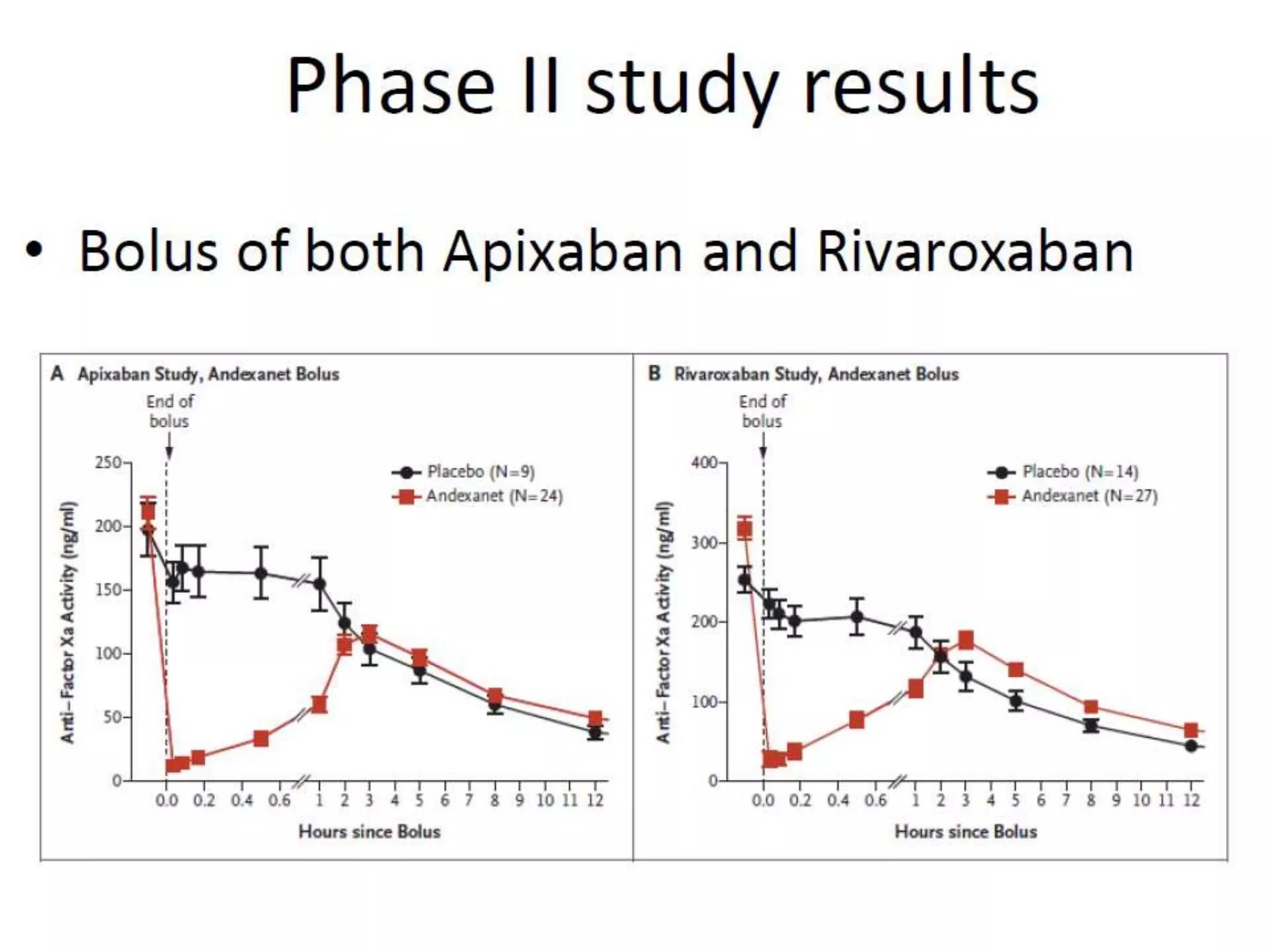
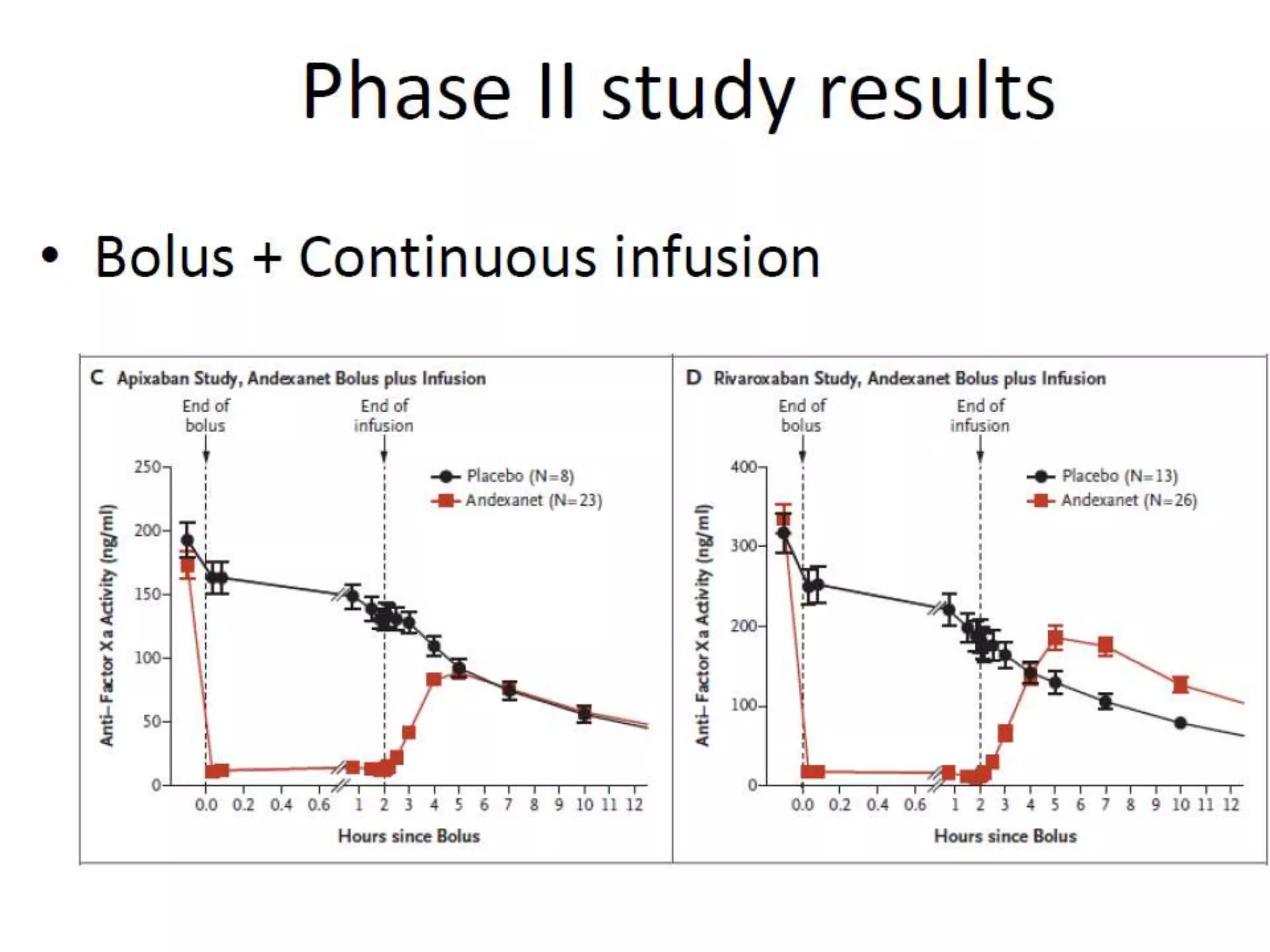
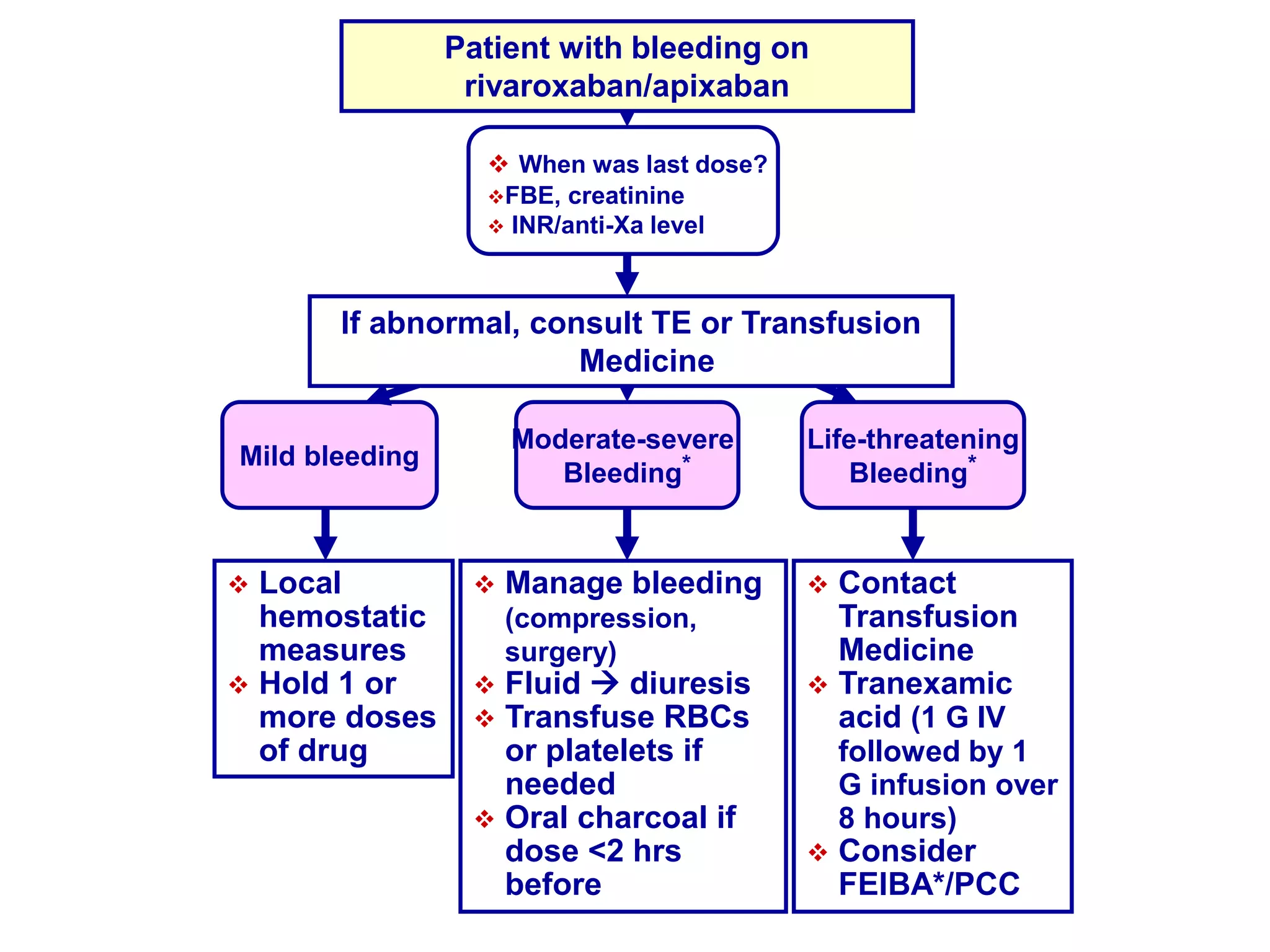
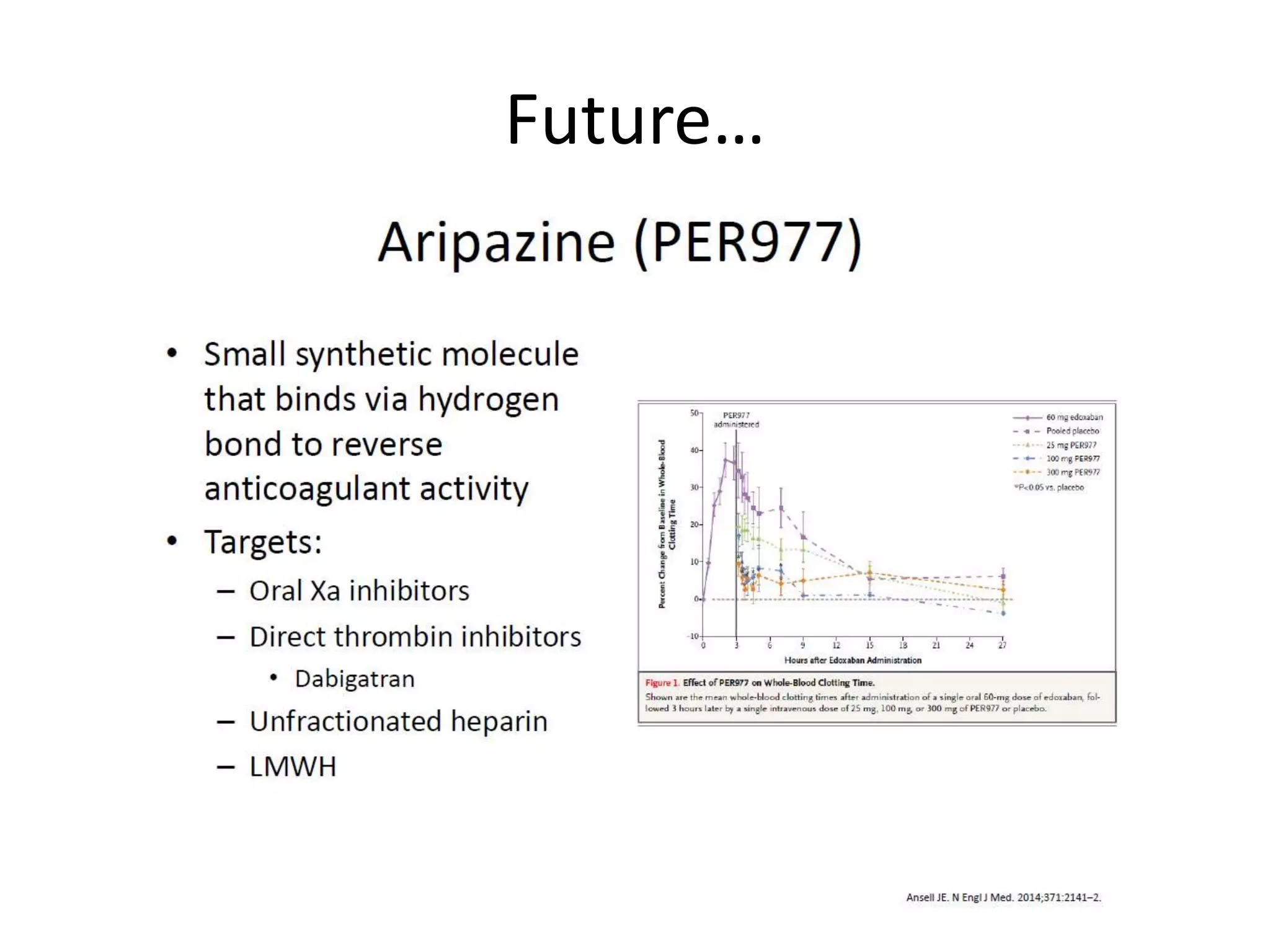
The document outlines key terminology related to novel oral anticoagulants (NOACs), direct oral anticoagulants (DOACs), and their management during bleeding events. It details protocols for preventing and treating bleeding, including the reversal of dabigatran and factor Xa inhibitors, with specific instructions for resuming anticoagulant therapy. The document emphasizes the importance of patient evaluation and laboratory monitoring in managing anticoagulation therapy and addressing bleeding complications.