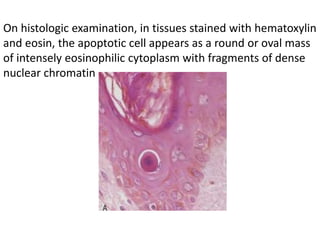
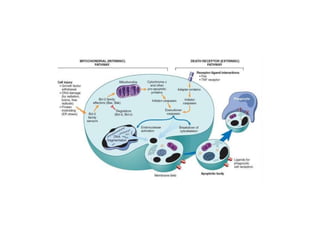
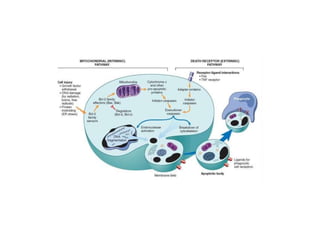
Apoptosis is a form of programmed cell death that is mediated by activation of caspases. It plays an important role in both physiological processes like development and pathological conditions like DNA damage. The key features of apoptosis include cell shrinkage, chromatin condensation, formation of apoptotic bodies, and phagocytosis of cell fragments without eliciting inflammation. The intrinsic and extrinsic pathways initiate apoptosis through an imbalance of survival and death signals within cells.