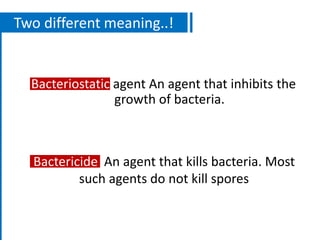
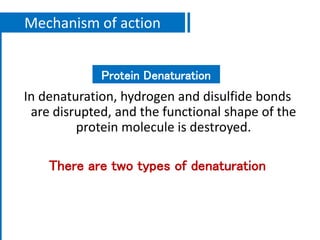
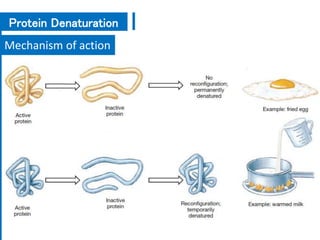
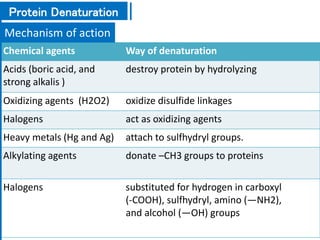
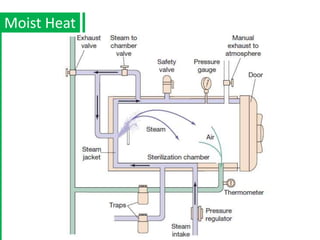
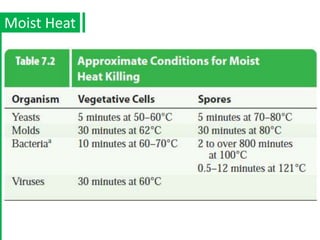
The document discusses antimicrobial methods for controlling microbial growth, highlighting the differences between sterilization, disinfection, antiseptics, and disinfectants. It describes various chemical agents and physical methods, including their mechanisms of action, effectiveness, and specific applications for controlling bacteria, fungi, and viruses. Additionally, it defines key terms related to antimicrobial agents and summarizes the factors influencing their efficacy.