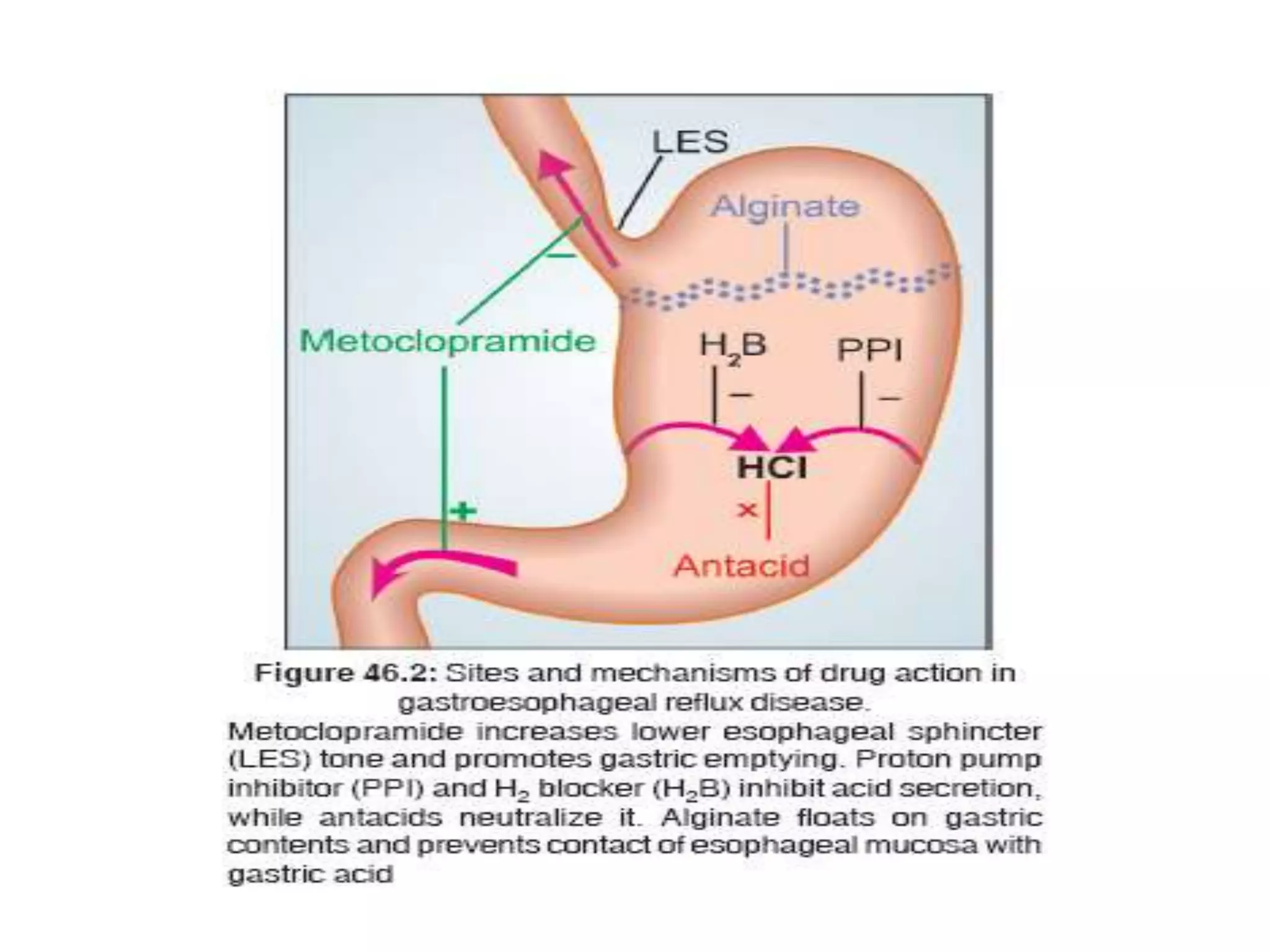
Antacids are substances that neutralize stomach acidity, providing relief from heartburn, indigestion, and acid reflux. They typically contain ingredients like aluminum, calcium, magnesium, or sodium bicarbonate and come in various forms, including liquids and chewable tablets. While generally safe, precautions are necessary for individuals with certain medical conditions, and misuse can lead to side effects such as constipation or diarrhea.