This document provides answers to self-tests and exercises from chapters 1-4 of an inorganic chemistry textbook.
For Chapter 1, it summarizes the answers to 20 self-test questions on topics like electron configurations, periodic trends, and nuclear reactions. It also provides answers to 27 exercises involving calculations related to these topics.
For Chapter 2, it summarizes the answers to 10 self-test questions on topics like molecular geometry and bonding theory. It also provides answers to 24 exercises involving identifying molecular geometries and describing bond types.
For Chapter 3, it summarizes the answers to 18 self-test questions on topics like crystal structures, lattice energies, and defects. It also provides answers to 24 exercises involving calculations
![Answers to self-tests and exercises
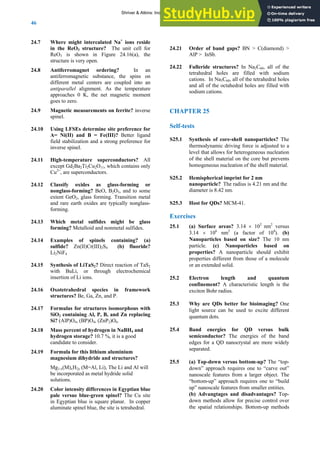
CHAPTER 1
Self-tests
S1.1
80
35 Br + 1
0 n 81
35 Br + γ
S1.2 d orbitals, 5 orbitals
S1.3 4
S1.4 3p
S1.5 The added p electron is in a different (p)
orbital, so it is less shielded.
S1.6 8 2
Ni :[Ar]3 4
d s , 2 8
Ni :[Ar]3d
+
S1.7 Period 4, Group 2, s block
S1.8 Going down a group the atomic radius
increases and the first ionization energy
generally decreases.
S1.9 Group 16. The first four electrons are
removed with gradually increasing values.
Removing the fifth electron requires a large
increase in energy, indicating breaking into a
complete subshell.
S1.10 Adding another electron to C would result in
the stable half filled p subshell.
S1.11 Cs+
Exercises
1.1 (a) 7
14
N+2
4
He→ 8
17
O+1
1
p +γ
(b) 6
12
C+1
1
p→ 7
13
N + γ
(c) 7
14
N+0
1
n→1
3
H+ 6
12
C
1.2 96
246
Cm+ 6
12
C→112
257
Uub+0
1
n
1.3 The higher value of I2 for Cr relative to Mn is
a consequence of the special stability of half-
filled subshell configurations and the higher
Zeff of a 3d electron verses a 4s electron.
1.4 22 4 25 1
10 2 12 0
Ne He Mg n
+ → +
1.5 4
9
Be+4
9
Be→ 6
12
C+2
4
He +20
1
n
1.6 Since helium-4 is the basic building block,
most additional fusion processes will produce
nuclei with even atomic numbers.
1.7 Take the summation of the rest masses of all
the nuclei of the products minus the masses of
the nuclei of the reactants. If you get a
negative number, energy will be released.
But what you have calculated is the mass
difference, which in the case of a nuclear
reaction is converted to energy.
1.8 0.25
1.9 –13.2 eV
1.10 1524nm, 1.524 X 104
cm-1
1.11
1
λ
= R
1
12
−
1
∞2
⎛
⎝
⎜
⎞
⎠
⎟ =1.0974X107m−1
1
λ
= R
1
12
−
1
42
⎛
⎝
⎜
⎞
⎠
⎟ =1.0288X107m−1
1
λ
= R
1
12
−
1
32
⎛
⎝
⎜
⎞
⎠
⎟ = 9.7547X106m−1
1
λ
= R
1
12
−
1
22
⎛
⎝
⎜
⎞
⎠
⎟ = 8.2305X106m−1
1.12 0 up to n-X
1.13 n2
1.14
N l ml Orbital
designation
Number
of
orbitals
2 1 +1, 0,
−1
2p 3
3 2 +2, +1,
…, −2
3d 5
4 0 0 4s 1
4 3 +3, +2,
…, −3
4f 7
1.15 n=5, l = 3, and ml = -3,-2,-1,0,1,2,3
1.16 Li: σ = Z – Zeff ; σ = 3-1.28 = 1.72
Be: σ = Z – Zeff ; σ = 4-1.19 = 2.09
B: σ = Z – Zeff ; σ = 5-2.42 = 2.58
C: σ = Z – Zeff ; σ = 6-3.14 = 2.86
N: σ = Z – Zeff ; σ = 7-3.83 = 3.17
O: σ = Z – Zeff ; σ = 8-4.45 = 3.55
F: σ = Z – Zeff ; σ = 9-5.10 = 3.90
1.17 The 1s electrons shield the positive charge
form the 2s electrons.
1
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/75/Answers-To-Self-Tests-And-Exercises-1-2048.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES
2
1.18 See Figs 1.11 through 1.16
1.19 See Table 1.6 and discussion.
1.20 Table 1.6 shows SR > Ba < Ra. Ra is
anomalous because of higher Zeff due to
lanthanide contraction.
1.21 Anomalously high value for Cr is associated
with the stability of a half filled d shell.
1.22 (a) [He]2s2
2p2
(b) [He]2s2
2p5
(c) [Ar]4s2
(d) [Ar]3d10
(e) [Xe]4f14
5d10
6s2
6p3
(f) [Xe]4f14
5d10
6s2
1.23 (a) [Ar]3d1
4s2
(b) [Ar]3d2
(c) [Ar]3d5
(d) [Ar]3d4
(e) [Ar]3d6
(f) [Ar]
(g) [Ar]3d10
4s1
(h) [Xe]4f 7
1.24 (a) Xe]4f14
5d4
6s2
(b) [Kr]4d6
(c) [Xe]4f6
(d) [Xe]4f7
(e) [Ar]
(f) [Kr]4d2
1.25 (a) S
(b) Sr
(c) V
(d) Tc
(e) In
(f) Sm
1.26 See Figure 1.4.
1.27 (a) I1 increases across the row except for a dip
at S; (b) Ae tends to increase except for Mg
(filled subshell), P (half filled subshell), and
at AR (filled shell).
1.28 Radii of Period 4 and 5 d-metals are similar
because of lanthanide contraction.
1.29 2s2
and 2p0
1.30
CHAPTER 2
Self-tests
S2.1
S2.2 (a) Angular
(b) square planar
S2.3 Linear
S2.4 S2
2–
: 1σg
2
2σu
2
3σg
2
1πu
4
2πg
4
;
Cl2
–
: 1σg
2
2σu
2
3σg
2
1πu
4
2πg
4
4σu
1
.
S2.5 1σg
2
2σu
2
3σg
2
1πu
4
2πg
4
S2.6 ½[2-2+4+2] = 3
S2.7 Bond order: C≡N, C=N, and C–N; Bond
strength: C≡N > C=N > C–N.
S2.8 If it contains 4 or fewer electrons.
S2.9 –21 kJ mol–1
S2.10 (a) +1/2
(b) +5
Exercises
2.1 (a) angular
(b) tetrahedral
(c) tetrahedral
2.2 (a) trigonal planar
(b) trigonal pyramidal
(c) square pyramidal
2.3 (a) T-shaped
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-2-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES 3
(b) square planar
(c) linear
2.4 (a)
I
Cl Cl
Cl Cl
(b)
S
F
F
F
F
1200
1800
2.5 (a) tetrahedral
(b) octahedral
2.6 (a) 176 pm
(b) 217 pm
(c) 221 pm
2.7 2(Si–O) = 932kJ > Si=O = 640kJ; therefore
two Si–O are preferred and SiO2 should (and
does) have four single Si–O bonds.
2.8 Multiple bonds are much stronger for period 2
elements than heavier elements
2.9 –483 kJ difference is smaller than expected
because bond energies are not accurate.
2.10 (a) 0
(b) 205 kJ mol-1
2.11 Difference in electronegativities are AB 0.5,
AD 2.5, BD 2.0, and AC 1.0. The increasing
covalent character AD < BD < AC < AB.
2.12 (a) covalent
(b) ionic
(c) ionic
2.13 (a) sp2
(b) sp3
(c) sp3
d or spd3
(d) p2
d2
or sp2
d.
2.14 (a) one
(b) one
(c) none
(d) two
2.15 (a) 1σg
2
2σu
2
(b) 1σg
2
2σu
2
1πu
2
(c) 1σg
2
2σu
2
1πu
4
3σg
1
(d) 1σg
2
2σu
2
3σg
2
1πu
4
2πg
3
2.16 The configuration for the neutral C2 would be
1σg
2
1σu
2
1πu
4
. The bond order would be ½[2-
2+4] = 2.
2.17 (a) 1σg
2
2σu
2
3σg
2
1πu
4
2πg
4
(b) 1
(c) There is no bond between the two atoms.
2.18 (a) 2
(b) 1
(c) 2
2.19 (a) +0.5
(b) –0.5
(c) +0.5
2.20
2.21 (a-c)
(d) Possibly stable in isolation (only bonding
and nonbonding orbitals are filled; not stable
in solution because solvents would have
higher proton affinity than He.
2.22 1
2.23 HOMO exclusively F; LUMO mainly S.
2.24 (a) electron deficient
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-3-320.jpg)

![ANSWERS TO SELF-TESTS AND EXERCISES 5
3.25 Yes.
3.26 A semiconductor is a substance with an
electrical conductivity that decreases with
increasing temperature. It has a small,
measurable band gap. A semimetal is a solid
whose band structure has a zero density of
states and no measurable band gap.
3.27 Ag2S and CuBr: p-type; VO2: n-type.
CHAPTER 4
Self-tests
S4.1 (a) HNO3 + H2O → H3O+
+ NO3
–
HNO3, acid. Nitrate ion, conjugate base. H2O,
base. H3O+
, conjugate acid.
(b) CO3
2–
+ H2O → HCO3
–
+ OH–
carbonate ion, base; hydrogen carbonate, or
bicarbonate, conjugate acid; H2O, acid;
hydroxide ion, conjugate base.
(c) NH3 + H2S → NH4
+
+ HS–
Ammonia, base; NH4
+
, conjugate acid;
hydrogen sulphide, acid; HS–
, conjugate base.
S4.2 What is the pH of a 0.10 M HF solution?
pH= 2.24
S4.3 Calculate the pH of a 0.20 M tartaric acid
solution?
pH=1.85
S4.4 Which solvent?
dimethylsulfoxide (DMSO) and ammonia.
S4.5 Is aKBrF4 an acid or a base in BrF3?
A base.
S4.6 Arrange in order of increasing acidity?
The order of increasing acidity is [Na(H2O)6]+
< [Mn(H2O)6]2+
< [Ni(H2O)6]2+
<
[Sc(H2O)6]3+
.
S4.7 Predict pKa values? (a) H3PO4 pKa ≈ 3. The
actual value, given in Table 4.1, is 2.1.
(b) H2PO4
–
pKa(2) ≈ 8. The actual value,
given in Table 4.1, is 7.4.
(c) HPO4
2–
pKa(3) ≈ 13. The actual value,
given in Table 4.1, is 12.7.
S4.8 What happens to Ti(IV) in aqueous
solution as the pH is raised?
Treatment with ammonia causes the
precipitation of TiO2. Further treatment with
NaOH causes the TiO2 to redissolve.
S4.9 Identify the acids and bases?
(a) FeCl3 + Cl–
→ [FeCl4]–
, acid is FeCl3,
base is Cl–
.
(b) I–
+ I2 → I3
–
, acid is I2, base is I–
.
S4.10 The difference in structure between
(H3Si)3N and (H3C)3N? The N atom of
(H3Si)3N is trigonal planar, whereas the N
atom of (H3C)3N is trigonal pyramidal.
S4.11 Draw the structure of BF3·OEt2?
Exercises
4.1 Sketch an outline of the s and p blocks of
the periodic table, showing the elements
that form acidic, basic, and amphoteric
oxides?
The elements that form basic oxides are in
plain type, those forming acidic oxides are in
outline type, and those forming amphoteric
oxides are in boldface type.
4.2 Identify the conjugate bases of the
following acids?
(a) [Co(NH3)5(OH2)]3+
, conjugate base is
[Co(NH3)5(OH)]2+
.
(b) HSO4
–
? The conjugate base is SO4
–
.
(c) CH3OH? The conjugate base is CH3O–
.
(d) H2PO4
–
? The conjugate base is HPO4
2–
.
(e) Si(OH)4? The conjugate base is
SiO(OH)3
–
.
(f) HS−
? The conjugate base is S2–
.
4.3 Identify the conjugate acids of the
following bases?
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-5-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES
6
(a) C5H5N (pyridine)? The conjugate acid
is pyridinium ion, C5H6N+
.
(b) HPO4
2–
? The conjugate acid is H2PO4
2–
.
(c) O2–
? The conjugate acid is OH–
.
(d) CH3COOH? The conjugate acid is
CH3C(OH)2
+
.
(e) [Co(CO)4]–
? The conjugate acid is
HCo(CO)4.
(f) CN–
? The conjugate acid is HCN.
4.4 Calculate the [H3O+
] and pH of a 0.10 M
butanoic acid solution? pH=2.85
4.5 What is the Kb of ethanoic acid?
Kb = 5.6 × 10–10
4.6 What is the Ka for C5H5NH+
?
Ka = 5.6 × 10–6
4.7 Predict if F
-
will behave as an acid or a
base in water?
F
-
will behave as a base in water.
4.8 What are the structures and the pKa values
of chloric (HClO3) and chlorous (HClO2)
acid?
Cl
O
O O
H Cl
O
O
H
chloricacid chlorousacid
Chloric acid, the predicted pKa = –2; actual
value = –1.
Chlorous acid, the predicted pKa = 3; actual
value = 2.
4.8 Which bases are too strong or too weak to
be studied experimentally? (a) CO3
2–
O2–
,
ClO4
–
, and NO3
–
in water?
CO3
2–
is of directly measurable base strength.
O2–
, is too strong to be studied experimentally
in water.
ClO4
2–
and NO3
–
are too weak to be studied
experimentally.
(b) HSO4
–
, NO3
–
, and ClO4
–
, in H2SO4?
HSO4
–
, not too strong to be studied
experimentally.
NO3
–
is of directly measurable base strength
in liquid H2SO4.
ClO4
–
, cannot be studied in sulfuric acid.
4.9 Is the –CN group electron donating or
withdrawing?
electron withdrawing
4.11 Is the pKa for HAsO4
2–
consistent with
Pauling’s rules? No. Pauling’s rules are
only approximate.
4.12 What is the order of increasing acid
strength for HNO2, H2SO4, HBrO3, and
HClO4?
the order is HClO4 > HBrO3 > H2SO4 >
HNO2.
4.13 Account for the trends in the pKa values of
the conjugate acids of SiO4
4–
, PO4
3–
, SO4
2–
,
and ClO4
–
?
The acidity of the four conjugate acids
increases in the order HSiO4
3–
< HPO4
2–
<
HSO4
–
< HClO4.
4.14 Which of the following is the stronger acid?
(a) [Fe(OH2)6]3+
or [Fe(OH2)6]2+
? The
Fe(III) complex, [Fe(OH2)6]3+
, is the stronger
acid.
(b) [Al(OH2)6]3+
or [Ga(OH2)6]3+
?
the aluminum-containing species is more
acidic.
(c) Si(OH)4 or Ge(OH)4?
Si(OH)4, is more acidic.
(d) HClO3 or HClO4?
HClO4 is a stronger acid.
(e) H2CrO4 or HMnO4?
HMnO4 is the stronger acid.
(f) H3PO4 or H2SO4?
H2SO4 is a stronger acid.
4.15 Arrange the following oxides in order of
increasing basicity? Al2O3, B2O3, BaO,
CO2, Cl2O7, and SO3?
order of increasing basicity is Cl2O7 < SO3 <
CO2 < B2O3 < Al2O3 < BaO.
4.16 Arrange the following in order of
increasing acidity? HSO4
−
, H3O+
, H4SiO4,
CH3GeH3, NH3, and HSO3F?
increasing acidity is NH3 < CH3GeH3 <
H4SiO4 < HSO4
–
< H3O+
< HSO3F.
4.17 Which aqua ion is the stronger acid, Na+
or
Ag+
? Ag+
(aq).
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-6-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES 7
4.18 Which of the following elements form oxide
polyanions or polycations? Al, As, Cu, Mo,
Si, B, Ti?
polycations: Al, Cu, and Ti.
polyoxoanions (oxide polyanions): Mo
polyoxoanions: As, B, and Si.
4.19 The change in charge upon aqua ion
polymerization?
Polycation formation reduces the average
positive charge per central M atom by +1 per
M.
4.20 Write balanced equations for the formation
of P4O12
4–
from PO4
3–
and for the formation
of [(H2O) 4Fe(OH)2Fe(OH2)4]4+
from
[Fe(OH2)6]3+
?
4PO4
3–
+ 8H3O+
→ P4O12
4–
+ 12H2O
2[Fe(OH2)6]3+
→
[(H2O)4Fe(OH)2Fe(OH2)4+
+ 2H3O+
4.21 More balanced equations?
(a) H3PO4 and Na2HPO4?
H3PO4 + HPO4
2-
ÅÆ 2H2PO4
-
(b) CO2 and CaCO3?
CO2 + CaCO3 + H2O Ca2+
+ 2HCO3
-
4.22 Give the equations for HF in H2SO4 and
HF in liquid NH3?
H2SO4 + HF ⇔ H3SO4
+
+ F-
NH3 + HF NH2
-
+ H2F+
4.23 Why is H2Se a stronger acid than H2S? As
you go down a family in the periodic chart,
the acidy of the homologous hydrogen
compounds increases.
4.24 Identifying elements that form Lewis
acids? All of the p-block elements except
nitrogen, oxygen, fluorine, and the lighter
noble gases form Lewis acids in one of their
oxidation states.
4.25 Identifying acids and bases: (a) SO3 + H2O
→ HSO4
–
+ H+
? The acids in this reaction
are the Lewis acids SO3 and H+
and the base
is the Lewis base OH–
.
(b) Me[B12]–
+ Hg2+
→ [B12] +
MeHg+
? The Lewis acid Hg2+
displaces the
Lewis acid [B12] from the Lewis base CH3
–
.
(c) KCl + SnCl2 → K+
+ [SnCl3]–
? The
Lewis acid SnCl2 displaces the Lewis acid K+
from the Lewis base Cl–
.
(d) AsF3(g) + SbF5(g) → [AsF2][SbF6]?
The very strong Lewis acid SbF5 displaces the
Lewis acid [AsF2]+
from the Lewis base F–
.
(e) EtOH readily dissolves in pyridine? A
Lewis acid–base complex formation reaction
between EtOH (the acid) and py (the base)
produces the adduct EtOH–py.
4.26 Select the compound with the named
characteristic? (a) Strongest Lewis acid:
BF3, BCl3, or BBr3? BBr3.
BeCl2 or BCl3? Boron trichloride.
B(n-Bu)3 or B(t-Bu)3? B(n-Bu)3.
(b) More basic toward BMe3: NMe3 or
NEt3? NMe3.
2-Me-py or 4-Me-py? 4-Me-py.
4.27 Which of the following reactions have Keq >
1? (a) R3P–BBr3 + R3N–BF3→ R3P–BF3 +
R3N–BBr3? <1
(b) SO2 + Ph3P–HOCMe3 → Ph3P–SO2 +
HOCMe3? > 1.
(c) CH3HgI + HCl → CH3HgCl + HI? <1
(d) [AgCl2]–
(aq) + 2 CN–
(aq) → [Ag (CN)2]–
(aq) + 2Cl–
(aq)? >1
4.28 Choose between the two basic sites in
Me2NPF2?
The phosphorus atom in Me2NPF2 is the
softer of the two basic sites, so it will bond
more strongly with the softer Lewis acid BH3
The hard nitrogen atom will bond more
strongly to the hard Lewis acid BF3.
4.29 Why is trimethylamine out of line?
Trimethyl amine is sterically large enough to
fall out of line with the given enthalpies of
reaction.
4.30 Discuss relative basicities? (a) Acetone and
DMSO?
DMSO is the stronger base regardless of how
hard or how soft the Lewis acid is. The
ambiguity for DMSO is that both the oxygen
atom and sulfur atom are potential basic sites.
(b) Me2S and DMSO? Depending on the
EA and CA values for the Lewis acid, either
base could be stronger.
4.31 Write a balanced equation for the
dissolution of SiO2 by HF?
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-7-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES
8
SiO + 6HF
2 2H O + H SiF
SiO2 + 4HF
2 2 6
or
2H2O + SiF4
Both a Brønsted acid–base reaction and a
Lewis acid–base reaction.
4.32 Write a balanced equation to explain the
foul odor of damp Al2S3? The foul odor
suggests H2S formation.
Al2S3 + 3H2O Al2O3 + 3H2S
4.33 Describe solvent properties? (a) Favor
displacement of Cl–
by I–
from an acid
center? If you choose a solvent that
decreases the activity of chloride relative to
iodide, you can shift the following
equilibrium to the right:
acid-Cl-
+ I- acid-I- + Cl-
(b) Favor basicity of R3As over
R3N? Alcohols such as methanol or ethanol
would be suitable.
(c) Favor acidity of Ag+
over Al3+
? An
example of a suitable solvent is diethyl ether.
Another suitable solvent is H2O.
(d) Promote the reaction 2FeCl3 + ZnCl2
→Zn2+
+ 2[FeCl4]−
? A suitable solvent is
acetonitrile, MeCN.
4.34 Propose a mechanism for the acylation of
benzene? An alumina surface, such as the
partially dehydroxylated one shown below,
would provide Lewis acidic sites that could
abstract Cl–
:
4.35 Why does Hg(II) occur only as
HgS? Mercury(II) is a soft Lewis acid, and
so is found in nature only combined with soft
Lewis bases, the most common of which is
S2–
.
4.36 Write Brønsted acid–base reactions in
liquid HF?
(a) CH3CH2OH? The balanced equation
is:
CH3CH2OH + HF CH3CH2OH2
+ + F-
(b) NH3? The equation is
(c) C6H5COOH?
C6H5COOH + HF C6H5COO- + H2F+
4.37 The dissolution of silicates by HF? both
4.38 Are the f-block elements hard? yes.
4.39 Calculate the enthalpy change for I2 with
phenol?
ΔfH
φ
= -20.0kJ/mol
CHAPTER 5
Self-tests
S5.1 Half-reactions and balanced reaction for
oxidation of zinc metal by permanganate
ions?
2[MnO4
−
(aq) + 8H+
(aq) + 5e–
→ Mn2+
(aq)
+ 4H2O(l)] reduction
5 [ Zn(s) → Zn2+
(aq) + 2e–
] oxidation
2MnO4
−
(aq) + 5Zn(s) + 16H+
(aq) →
5Zn2+
(aq) + 2Mn2+
(aq) + 8H2O(l)
S5.2 Does Cu metal dissolve in dilute HCl? No.
S5.3 Can Cr2O7
2–
be used to oxidize Fe2+
, and
would Cl–
oxidation be a problem? Yes.
Cl-
oxidation is not a problem.
S5.4 Fuel cell emf with oxygen and hydrogen
gases at 5.0 bar?
E = 1.25 V.
S5.5 The fate of SO2 emitted into clouds? The
aqueous solution of SO4
2–
and H+
ions
precipitates as acid rain.
S5.6 Can Fe2+
disproportionate under standard
conditions? No.
S5.7 bpy binding to Fe(III) or Fe(II)? Fe(II)
preferentially.
S5.8 Potential of AgCl/Ag,Cl–
couple?
Eox= – 1.38 V
S5.9 Latimer diagram for Pu? (a) Pu(IV)
disproportionates to Pu(III) and Pu(V) in
aqueous solution; (b) Pu(V) does not
disproportionate into Pu(VI) and Pu(IV).
S5.10 Frost diagram for thallium in aqueous
acid?
N H 4
+ + F -
N H 3 + H F
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-8-320.jpg)

![ANSWERS TO SELF-TESTS AND EXERCISES
10
2MnO4
−
(aq) + 5H2SO3(aq) + H+
(aq) →
2Mn2+
(aq) +5HSO3
−
(aq) + 3H2O(l)
The potential decreases as the pH increases.
5.7 Write the Nernst equation for (a) The
reduction of O2?
Q = 1/(p(O2)[H+
]4
)
and
E = Eº – [(0.059V)/4][log(1/(p(O2)[H+
]4
)]
(b) The reduction of Fe2O3(s)?
Q = 1/[H+
]6
and E = Eº – (RT/nF)(13.8 pH)
5.8 Using Frost diagrams? (a) What happens
when Cl2 is dissolved in aqueous basic
solution? Cl2 is thermodynamically
susceptible to disproportionation to Cl–
and
ClO4
–
when it is dissolved in aqueous base.
The oxidation of ClO–
is slow, so a solution of
Cl–
and ClO–
is formed when Cl2 is dissolved
in aqueous base.
(b) What happens when Cl2 is dissolved in
aqueous acid solution? Cl2 will not
disproportionate. Cl2 is thermodynamically
capable of oxidizing water.
(c) Should HClO3 disproportionate in
aqueous acid solution? Kinetic.
5.9 Write equations for the following
reactions: (a) N2O is bubbled into aqueous
NaOH solution?
5N2O(aq) + 2OH–
(aq) → 2NO3
–
(aq) +
4N2 (g) + H2O(l)
(b) Zinc metal is added to aqueous acidic
sodium triiodide?
Zn(s) + I3
–
(aq) → Zn2+
(aq) + 3I–
(aq)
(c) I2 is added to excess aqueous acidic
HClO3?
3I2(s) + 5ClO3
–
(aq) + 3H2O(l) → 6IO3
–
(aq) + 5Cl–
(aq) + 6H+
(aq)
5.10 Electrode potential for Ni2+
/Ni couple at pH
= 14? E =– 0.21 V
5.11 Will acid or base most favour the following
half-reactions? (a) Mn2+
→ MnO4
–
? Base
(b) ClO4
–
→ ClO3
–
? Acid
(c) H2O2 → O2? Base
(d) I2 → 2I–
? Acid or base, no difference.
5.12 Determine the standard potential for the
reduction of ClO4
–
to Cl2? 1.392 V
5.13 Calculate the equilibrium constant for
Au+
(aq) + 2CN–
(aq) → [Au(CN)2]–
(aq)?
K = 5.7 × 1038
5.14 Find the approximate potential of an
aerated lake at pH = 6, and predict the
predominant species? (a) Fe? 0.5 – 0.6 V
(b) Mn? E = 0.55 V
(c) S? At pH 0, 0.387 V. At pH 14, SO4
2–
would again predominate. HSO4
–
is the
predominant sulfur species at pH 6.
5.15 Frost diagram and standard potential for
the HSO4
−
/S8(s) couple? 0.387 V
5.16 Equilibrium constant for the reaction
Pd2+
(aq) + 4 Cl–
(aq) ≡ [PdCl4]2–
(aq) in 1 M
HCl(aq)? K = 4.37 × 1010
5.17 Reduction potential for MnO4
–
to MnO2(s)
at pH = 9.00? E = 0.98 V
5.18 Tendency of mercury species to act as an
oxidizing agent, a reducing agent, or to
undergo disproportionation? Hg2+
and
Hg2
2+
are both oxidizing agents. None of
these species are likely to be good reducing
agents. Hg2
2+
is not likely to undergo
disproportionation.
5.19 Thermodynamic tendency of HO2 to
undergo disproportionation? E = +1.275 V.
(is positive), HO2 will undergo
disproportionation.
5.20 Dissolved carbon dioxide corrosive towards
iron? Carbon dioxide and water generate
carbonic acid which encourages the corrosion
process by lowering solution pH.
5.21 What is the maximum E for an anaerobic
environment rich in Fe2+
and H2S? –0.1 V.
5.22 How will edta4–
complexation affect M2+
→
M0
reductions? The reduction of a M(edta)2–
complex will be more difficult than the
reduction of the analogous M2+
aqua ion.
5.23 Which of the boundaries depend on the
choice of [Fe2+
]? Any boundary between a
soluble species and an insoluble species will
change as the concentration of the soluble
species changes. The boundaries between the
two soluble species, and between the two
insoluble species, will not depend on the
choice of [Fe2+
].
5.24 Under what conditions will Al reduce
MgO? Above about 1400ºC.
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-10-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES 11
CHAPTER 6
Self-tests
S6.1 Sketch the S4 axis of an NH4
+
ion. How
many of these axes are there in the ion?
Three S4 axes.
S6.2 (a) BF3 point group? D3h.
(b) SO4
2–
point group? Td.
S6.3 Symmetry species of all five d orbitals of
the central Xe atom in XeF4 (D4h, Fig. 6.3)?
dx2-y2 is B1g;
dxy is B2g;
dxz and dyz are Eg;
dz2 is A1g.
S6.4 What is the maximum possible degeneracy
for an Oh molecule? 3.
S6.5 A conformation of the ferrocene molecule
that lies 4 kJ mol–1
above the lowest energy
configuration is a pentagonal antiprism. Is
it polar? No.
S6.6 Is the skew form of H2O2 chiral? Yes.
S6.7 Can the bending mode of N2O be Raman
active? Yes.
S6.8 Confirm that the symmetric mode is Ag?
D2h character table, which is the Ag symmetry
type.
S6.9 Show that the four CO displacements in
the square-planar (D4h) [Pt(CO)4]2+
cation
transform as A1g + B1g + Eu. How many
bands would you expect in the IR and
Raman spectra for the [Pt(CO)4]2+
cation?
The reducible representation:
D4h E 2C4 C2
2C2
’
2C2
″
i 2S4
σh 2σv 2σd
Γ3N 4 0 0 2
0 0 0 4 2 0
Reduces to A1g + B1g + Eu
A1g + B1g are Raman active. Eu is IR active.
S6.10 Orbital symmetry for a tetrahedral array
of H atoms in methane? A1
S6.11 Orbital symmetry for a square-planar
array of H atoms? B2g.
S6.12 Which Pt atomic orbitals can combine with
which of these SALCs? The atomic orbitals
much have matching symmetries to
generate SALCs. 5s and 4dz2 have A1g
symmetry; the dx2-y2 has B1g symmetry; and
5px and 5py have Eu symmetry.
S6.13 Predict how the IR and Raman spectra of
SF5Cl differ from that of SF6?
SF6 has Oh symmetry. Analysis of the
stretching vibrations leads to:
Γstr = A1g (Raman, polarized) + Eg
(Raman) + T1u (IR).
SF5Cl has C4v symmetry. Analysis of the
stretching vibrations leads to:
Γstr = 3A1 (IR and Raman, polarized)
+ 2B1 (Raman) + E (IR, Raman).
S6.14 Symmetries of all the vibration modes of
[PdCl4]2-
? A1g + B1g + B2g + A2u + B2u + 2Eu
S6.15 SALCs for sigma bonding in O? A1g + Eg
+T1u.
Exercises
6.1 Symmetry elements? (a) a C3 axis and a σv
plane in the NH3 molecule?
N
H
H
H
H
N
H
H
C3 σv
(b) a C4 axis and a σh plane in the square-
planar [PtCl4]2–
ion?
Pt
Cl
Cl Cl
Cl
Pt
Cl
Cl Cl
Cl
C4 σh
6.2 S4 or i? (a) CO2? i
(b) C2H2? i.
(c) BF3? neither.
(d) SO4
2–
? three different S4.
6.3 Assigning point groups: (a) NH2Cl? Cs
(b) CO3
2–
? D3h
(c) SiF4? Td
(d) HCN? C∞v.
(e) SiFClBrI? C1.
(f) BrF4
–
? D4h.
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-11-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES
12
6.4 How many planes of symmetry does a
benzene molecule possess? What chloro-
substituted benzene has exactly four planes
of symmetry? 7,and C6H3Cl3.
6.5 The symmetry elements of orbitals? (a) An
s orbital? Infinite number of Cn axes, plus
an infinite number of mirror planes of
symmetry, plus center of inversion, i.
(b) A p orbital? An infinite number of mirror
planes that pass through both lobes and
include the long axis of the orbital. In
addition, the long axis is a Cn axis, where n
can be any number from 1 to ∞.
(c) A dxy orbital? Center of symmetry, three
mutually perpendicular C2 axes, three
mutually perpendicular mirror planes of
symmetry, two planes that are rotated by 45º
about the z axis from the xz plane and the yz
plane.
(d) A dz2 orbital? In addition to the
symmetry elements possessed by a p orbital:
(i) a center of symmetry, (ii) a mirror plane
that is perpendicular to the C∞ axis, (iii) an
infinite number of C2 axes that pass through
the center of the orbital and are perpendicular
to the C∞ axis, and (iv) an S∞ axis.
6.6 SO3
2–
ion? (a) Point group? C3v
(b) Degenerate MOs? 2
(c) Which s and p orbitals have the
maximum degeneracy? 3px and 3py orbitals
are doubly degenerate.
6.7 PF5? (a) Point group? D3h.
(b) Degenerate MOs? 2.
(c) Which p orbitals have the maximum
degeneracy? 3px and 3py atomic orbitals are
doubly degenerate
6.8 AsCl5 Raman spectrum consistent with a
trigonal bipyamidal geometry? No.
6.9 Vibrational modes of SO3? (a) In the plane
of the nuclei? 5
(b) Perpendicular to the molecular plane?
1
6.10 Vibrations that are IR and Raman active?
(a) SF6? None.
(b) BF3? The E′ modes are active in both IR
and Raman.
6.11 Vibrations of a C6v molecule that are
neither IR nor Raman active? Any A2, B1,
or B2 vibrations of a C6v molecule will not be
observed in either the IR spectrum or the
Raman spectrum.
6.12 [AuCl4]−
ion? Γ of all 3N displacements
and irreducible representations?
A1g + A2g + B1g + B2g + Eg + 2A2u + B2u + 3Eu
6.13 IR and Raman to distinguish between: (a)
planar and pyramidal forms of PF3, (b)
planar and 90o
-twisted forms of B2F4 (D2h
and D2d respectively)?
(a) Planar PF3, D3h, vibrations are: A1’
(Raman, polarized) + 2E’ (IR and Raman) +
A2” (IR).
Pyramidal PF3, C3v, vibrations are: 2A1 (IR
and Raman, polarized) + 2E’ (IR and Raman)
(b) For the planar form of B2F4 (D2h):
The vibrations are:
3Ag (Raman, polarized) + 2B2g (Raman) +
B3g (Raman) + Au(inactive) + 2B1u (IR) +
B2u (IR) + 2B3u (IR).
For the 90o
-twisted form of B2F4 (D2d)
The vibrations are: 3A1 (Raman, polarized) +
B1 (Raman) + 2B2 (IR and Raman ) + 3E (IR
and Raman).
6.14 (a) Take the 4 hydrogen 1s orbitals of CH4
and determine how they transform under
Td. (b) Confirm that it is possible to reduce
this representation to A1 + T2. (c) Which
atomic orbitals on C can form MOs with
H1s SALCs?
Using symmetry Td, Γ3N reduces to: A1 + T2.
The MOs would be constructed from SALCs
with H1s and 2s and 2p atomic orbitals on C.
6.15 Use the projection operator method to
construct the SALCs of A1 + T2 symmetry
that derive from the four H1s orbitals in
methane..
s = (1
/2)(ϕ1 + ϕ2 + ϕ3 + ϕ3) (= A1)
px = (1
/2)(ϕ1 – ϕ2 + ϕ3 – ϕ3) (= T2)
py = (1
/2)(ϕ1 – ϕ2 – ϕ3 + ϕ3) (= T2)
pz = (1
/2)(ϕ1 + ϕ2 – ϕ3 – ϕ3) (= T2)
SALCs for σ-bonds
(a) BF3?
(1
/√3)(ϕ1 + ϕ2 + ϕ3) (= A1’)
(1
/√6)(2ϕ1 – ϕ2 – ϕ3) and (1
/√2)(ϕ2 – ϕ3) (=
E’)
(b) PF5?
(axial F atoms are ϕ4 + ϕ5)
(1
/√2)(ϕ4 + ϕ5) (= A1’)
(1
/√2)(ϕ4 − ϕ5) (= A2”)
(1
/√3)(ϕ1 + ϕ2 + ϕ3) (= A1’)
(1
/√6)(2ϕ1 – ϕ2 – ϕ3) and (1
/√2)(ϕ2 – ϕ3) (=
E’)
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-12-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES 13
CHAPTER 7
Self-tests
S7.1 Give formulas corresponding to the
following names? (a) Cis-
diaquadichloroplatinum(II)? cis-
[PtCl2(OH2)2],
trans-diaquadichloroplatinum(II), trans-
[PtCl2(OH2)2].
(b) Diamminetetra(isothiocyanato)
chromate(III)?
[Cr(NCS)4(NH3)2] –
. ,can exist as, cis-
[Cr(NCS)4(NH3)2] –
or trans-
[Cr(NCS)4(NH3)2] –
.
(c) Tris(ethylenediamine)rhodium (III)?
[Rh(en)3]3+
.
(d) Bromopentacarbonylmanganese
(I)? [MnBr (CO)5].
(e) Chlorotris(triphenylphosphine)rhodium
(I)? [RhCl(PPh3)3].
S7.2 What type of isomers are possible for
[Cr(NO2)2•6H2O]? The hydrate isomers
and linkage isomers of the NO2 group. Also,
[Cr(ONO)(H2O)5]NO2 •H2O.
S7.3 Identifying isomers? Note that the two
phosphine ligands in the trans isomer are
related, therefore, they exhibit the same
chemical shift.
S7.4 Sketches of the mer and fac isomers of
[Co(gly)3]?
S7.5 Which of the following are chiral? (a) cis-
[CrCl2 (ox)2]3–
? Chiral .
(b) trans-[CrCl2(ox)2]3–
? Not chiral.
(c) cis-[RhH(CO)(PR3)2]? Not chiral.
S7.6 Calculate all of the stepwise formation
constants? Kf1 = 1 X 105
. Kf2 will be 30%
less or 30000, Kf3 = 9000, Kf4 = 2700, Kf5 =
810, and finally Kf6 = 243.
Exercises
7.1 Name and draw the structures of the
complexes? (a) [Ni(CO)4]? Nickel
tetracarbonyl or tetracarbonyl nickel(0).
CO
Ni
OC
CO
CO
(b) [Ni(CN)4]2–
? Tetracyanonickelate (II).
2-
Ni
NC
NC CN
CN
(c) [CoCl4]2–
? Tetrachlorocobaltate (II)
2-
Cl
Co
Cl
Cl
Cl
(d) [Mn(NH3)6]2+
?
Hexaamminemanganesium (II)
2
+
M
n
H
3
N
H
3
N N
H
3
N
H
3
N
H
3
N
H
3
7.2 Write the formulas for the following
complexes?
(a) [CoCl(NH3)5]Cl2
(b) [Fe(OH2)6](NO3)3
(c) cis-[FeCl2(en)2]
(d) [Cr(NH3)5μ–OH–Cr(NH3)5]Cl5
7.3 Name the following complexes?
(a) cis-[CrCl2(NH3)4]+
? cis-
tetra(ammine)di(chloro)chromium(III)
(b) trans-[Cr(NCS)4(NH3)2]
-
? trans-
di(ammine)tetrakis(isothiocyanato)chromate
(III)
(c) [Co(C2O4)(en)2]+
?
bis(ethylenediamine)oxalatocobalt(III).
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-13-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES
14
7.4 Four-coordinate complexes? (a) Sketch the
two observed structures?
(b) Isomers expected for MA2B2?
tetrahedral complex, no isomers, for a square-
planar complex, two isomers, cis and trans.
7.5 For five-coordinate complexes, sketch the
two observed structures?
7.6 Six-coordinate complexes? (a) Sketch the
two observed structures?
(b) Which one of these is rare? Trigonal
prism.
7.7 Explain the difference between
monodentate, bidentate, and
quadridentate? A monodentate ligand can
bond to a metal atom only at a single atom, a
bidentate ligand can bond through two atoms,
a quadridentate ligand can bond through four
atoms.
7.8 What type of isomers do you get with
ambidentate ligands? linkage isomers.
7.9 Which ligand could act like a chelating
ligand? (a) Triphenylphosphite, no/
(b) Bis(dimethyl)phosphino ethane (dmpe)
yes.
(c) Bipyridine (bipy), yes.
(d) Pyrazine, no.
7.10 Draw structures of complexes that contain
the ligands (a) en, (b) ox, (c) phen, and (d)
edta? .
M
L L
L L
M
L
L
L
L
tetrahedral square plana
[Mg(edta)(OH2)]2–
7.11 What types of isomers are [RuBr(NH3)5]Cl
and [RuCl(NH3)5]Br? Ionization isomers.
7.12 Which complexes have isomers?
[CoBrClI(OH2)]
7.13 Which complexes have isomers?
(a) [Pt(ox)(NH3)2] no isomers
(b) [PdBrCl(PEt3)2] has two isomers.
(c) [IrHCO(PR3)2] has two isomers.
(d) [Pd(gly)2] has two isomers.
7.14 How many isomers are possible for the
following complexes?
(a) [FeCl(OH2)5]2+
? None.
(b) [IrCl3(PEt3)2]? 2
(c) [Ru(biby)3]2+
? 2
(d) [CoCl2(en)(NH3)2]+
? 4
(e) [W(CO)4(py)2] 2
r
M
A
A
E
E
E
Trigonal Bipyramidal
A = axial ligands
E = equatorial ligands
M
A
B
B
B B
Square based pyramid
A = axial
B = basal
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-14-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES 15
7.15 Draw all possible isomers for [MA2BCDE]?
Including optical isomers, 15 isomers are
possible! .
M
A
E
C D
A
B
M
A
D
E C
A
B
M
A
C
D E
A
B
M
A
A
D C
E
B
M
A
A
C D
E
B
M
A
A
D E
C
B
M
A
C
B D
A
E
M
A
B
E C
A
D
M
A
E
D B
A
C
7.16 Which of the following complexes are
chiral? (a) [Cr(ox)3]3–
? Chiral
(b) cis-[PtCl2(en)]? Chiral (The en is not
planar).
(c) cis-[RhCl2(NH3)4]+
? not chiral.
(d) [Ru(bipy)3]2+
? chiral
(e) fac-[Co(NO2)3(dien)]? Not chiral.
(f) mer-[Co(NO2)3(dien)]? Chiral (dien is
not planar).
7.17 Which isomer, Λ or Δ, is the complex
Mn(acac)3, shown in the exercise? The Λ
isomer.
7.18 Draw both isomers, Λ or Δ, of the complex
[Ru(en)3]+2
?
7.19 Suggest a reason why Kf5 is so different?
Because of a change in coordination.
7.20 Compare these values with those of
ammonia given in exercise 7.19 and suggest
why they are different? The chelate effect.
CHAPTER 8
Self-tests
S8.1 Main features of the CrO2 powder XRD
pattern? XRD pattern for CrO2 will show
identical reflections to those of rutile TiO2 but
shifted to slightly higher diffraction angles.
S8.2 TiO2 in sunscreens?
Titania articles absorb this ultraviolet
radiation
S8.3 Molecular shape and vibrational modes for
XeF2? Trigonal bipyramidal, 4 total
vibrational modes.
S8.4 (a) 77
Se-NMR spectrum consists of a triplet
of triplets? The triplet of triplets.
(b) Proton resonance of the hydrido ligand
consist of eight equal intensity lines? yes.
S8.5 EPR signal of new material arises from W
sites?
14% of naturally occurring tungsten is 183
W,
which has I = ½. Thus, the signal is split into
2 lines.
S8.6 Isomer shift for iron in Sr2FeO4? The
Smaller and less positive.
S8.7 Why does the mass spectrum of ClBr3 have
five peaks separated by 2 u? Halogen
isomers.
Exercises
8.1 How would you determine crystalline
components in mineral sample? Powder X-
ray diffraction.
8.2 Why are there no diffraction maxima in
borosilicate glass? Glass has no long-range
periodicity or order.
8.3 What is the minimum size of a cubic
crystal that can be studied? 0.5 μm by 0.5
μm by 0.5 μm.
Ru
N
N
N
N
N
N
Ru
N
N
N
N
N
N
Δ isomer
Λ isomer
8.4 Wavelength of neutron at 2.20 km/s? 1.80 ×
10–12
m or 180 pm.
8.5 Order of stretching frequencies? The
smaller effective mass of the oscillator for
CN−
causes the molecule to have the higher
stretching frequency. The bond order for NO
is 2.5, and N is heavier than C, hence CO has
a higher stretching frequency than NO.
8.6 Wavenumber for O–O in O2
+
? In the region
of 1800 cm1
.
8.7 UV photoelectron spectrum of NH3? The
band at 11 eV is due to the lone pair and the
pyramidal angle. The ionised molecule has
greater planarity, thus the long progression.
8.8 Raman bands assignments? N(SiH3)3 is
planar. N(CH3) 3 is pyramidal.
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-15-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES
16
8.9 Single 13
C peak in NMR? Chemically
distinct carbonyls are exchanging position
sufficiently quickly.
8.10 Form of 19
F-NMR and 77
Se-NMR spectra
of 77
SeF4? 19
F NMR spectrum reveals two
1:3:3:1 quartets. The 77
Se-NMR spectrum is a
triplet of triplets.
8.11 NMR spectral features for XeF5
−
? All 5 of
the F atoms are chemically equivalent.
Approximately 25% is present as 129
Xe, I =
1/2, and in this case the 19
F resonance is a
doublet. The final result is a composite: two
lines of 12.5% intensity from the 19
F coupled
to the 129
Xe, and one remaining line of 75%.
8.12 g-values? 1.94, 1.74, and 1.56.
8.13 Slower process, NMR or EPR? NMR.
8.14 Differences in EPR spectrum for d-metal
with one electron in solution versus frozen?
In aqueous solution at room temperature,
molecular tumbling removes the effect of the
g-value anisotropy. In frozen solution, g-
value anisotropy can be observed.
8.15 Isomer shift for iron in BaFe(VI)
O4? A
positive shift for Fe(VI) well below +0.2 mm
s-1
.
8.16 Charge on Fe atoms in Fe4[Fe(CN)6]3? EPR
and Mössbauer.
8.17 No quadrupole splitting in Mössbauer
spectrum of SbF5? The geometry must be
close to cubic in the solid state.
8.18 No peak in the mass spectrum of Ag at 108
u? Two isotopes, 107
Ag (51.82%) and 109
Ag
(48.18%). Compounds that contain silver will
have two mass peaks.
8.19 Peaks in mass spectrum of
Mo(C6H6)(CO)3? 258, 230, 200, 186, and
174.
8.20 Cyclic voltammogram of Fe(III) complex?
The complex undergoes a reversible one-
electron reduction with a reduction potential
of 0.21 V. Above 0.720 V the complex is
oxidized.
8.21 Zeolite of composition CaAl2Si6O16.nH2O,
determine n.? n=7.2 As an integer, n = 7.
8.22 Ratio of cobalt to acetylacetonate in the
product? The ratio is 3:1.
CHAPTER 9
Self-tests
S9.1 Found in aluminosilicate minerals or
sulfides?
Cd and Pb will be found as sulfides.
Rb and Sr can be found in aluminosilicate
minerals.
Cr and Pd can be found in both oxides and
sulfides.
S9.2 Sulfur forms catenated polysulfides
whereas polyoxygen anions are unknown?
Owing to a strong tendency to form strong
double bonds, it is more likely that
polyoxygen anions will form pi bonds that
limit extended bonding owing to restrictions
on pi orbital overlap through multiple
bridging centres.
S9.3 Shape of XeO4 and identify the Z + 8
compound with the same structure?
A tetrahedral geometry. The same structure is
SmO4.
S9.4 Comment on ΔfHө
values? It is evident from
the values that as we move down the group,
steric crowding of the fluorines is minimized.
S9.5 Further data useful when drawing
comparisons with the value for V2O5? We
would have to know the products formed
upon decomposition.
Exercises
9.1 Maximum stable oxidation state? (a) Ba;
+2, (b) As; +5, (c) P; +5, (d) Cl; +7.
9.2 Form saline hydrides, oxides and
peroxides, and all the carbides react with
water to liberate a hydrocarbon?
the alkaline earth metals or Group 2 elements.
9.3 Elements vary from metals through
metalloids to non-metals; form halides in
oxidation states +5 and +3 and toxic
gaseous hydrides? Elements in Group 15.
9.4 Born–Haber cycle for the formation of the
hypothetical compound NaCl2? Which
thermochemical step is responsible for the
fact that NaCl2 does not exist?
The second ionization energy of
sodium is 4562 kJ mol-1
and is responsible for
the fact that the compound does not exist.
9.5 Inert pair effect beyond Group 15? The
relative stability of an oxidation state in which
the oxidation number is 2 less than the group
number is an example of the inert pair effect.
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-16-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES 17
9.6 Ionic radii, ionization energy, and metallic
character? Metallic character, ionic radii
decrease across a period and down a group.
Ionization energy increases across a period
and decreases down a group.
9.7 Names of ores? (a) Mg; MgCO3 magnesite,
(b) Al; Al2O3 bauxite, and (c) Pb; PbS galena.
9.8 Identify the Z + 8 element for P.
Similarities? V (vanadium).
9.9 Calculate ΔfHө
for SeF6?
ΔfHө
= −1397 kJ mol-1
.
CHAPTER 10
Self-tests
S10.1 Which of the following CH4, SiH4,, or GeH4
would best H+
or H
-
donor?
CH4, the strongest Bronsted acid. GeH4
would be the best hydride donor.
S10.2 Reactions of hydrogen compounds?
(a) Ca(s) + H2(g) → CaH2(s).
(b) NH3(g) + BF3(g) → H3N–BF3(g).
(c) LiOH(s) + H2(g) → NR.
S10.3 A procedure for making Et3MeSn?
2Et3SnH + 2Na → 2Na+
Et3Sn–
+ H2
Na+
Et3Sn–
+ CH3Br → Et3MeSn + NaBr
Exercises
10.1 Where does Hydrogen fit in the periodic
chart? (a) Hydrogen in group 1? Hydrogen
has one valence electron like the group 1
metals and is stable as H+
, especially in
aqueous media.
(b) Hydrogen in group 17? Hydrogen can
fill its 1s orbital and make a hydride H–
. The
halogens are diatomic gases just like
hydrogen, but chemically it fits well in both
group 1 and group 17.
(c) Hydrogen in group 14? There is no
reason for hydrogen to be placed in this
group.
10.2 Low reactivity of hydrogen? Hydrogen
exists as a diatomic molecule (H2). It has a
high bond enthalpy. It also only has two
electrons shared between two protons.
10.3 Assign oxidation numbers to elements?
(a) H2S? H = +1, S = –2.
(b) KH? H = –1, K = +1.
(c) [ReH9]2–
? H = –1, Re = +7.
(d) H2SO4? H = +1, O = –2, S = +6.
(e) H2PO(OH)? H bonded to an oxygen
atom = +1. H bonded to the phosphorus atom?
If they are assigned an oxidation number of
+1, and O = –2, then P = +1.
10.4 Preparation of hydrogen gas?
(i) CH4(g) + H2O → CO(g) + 3H2(g) (1000°C)
(ii) C(s) + H2O → CO(g) + H2(g) (1000°C)
(iii) CO(g) + H2O → CO2(g) + H2 (g)
10.5 Properties of hydrides of the elements? (a)
Position in the periodic table? See Figure
10.2.
(b) Trends in ΔfGº? See Table 10.1.
(c) Different molecular
hydrides? Molecular hydrides are found in
groups 13/III through 17/VII.
10.6 What are the physical properties of water
without hydrogen bonding? It most likely
would be a gas at room temperature; ice
would be denser than water.
10.7 Which molecule has the stronger hydrogen
bonds? S–H···O has a weaker hydrogen bond
than O–H···S.
10.8 Name and classify the following?
(a) BaH2? barium hydride.
(b) SiH4? silane.
(c) NH3? ammonia,
(d) AsH3? Arsine.
(e) PdH0.9? palladium hydride.
(f) HI? hydrogen iodide.
10.9 Chemical characteristics of hydrides?
(a) Hydridic character? Barium hydride
(b) Brønsted acidity? Hydrogen iodide.
(c) Variable composition? PdH0.9 .
(d) Lewis basicity? Ammonia.
10.10 Phases of hydrides of the elements? BaH2
and PdH0.9 are solids, none is a liquid, and
SiH4, NH3, AsH3, and HI are gases.
10.11 Structures of H2Se, P2H4, and H3O+
? The
Lewis structures of these three species are:
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-17-320.jpg)


![ANSWERS TO SELF-TESTS AND EXERCISES
20
12.6 Explain why Group 1 hydroxides are much
more corrosive to metals than Group 2
hydroxides?
Group 1 hydroxides are more soluble than
group 2 hydroxides, and therefore have higher
OH−
concentrations.
12.7 Which of the salts MgSeO4 or BaSeO4 would
be expected to be more soluble in water?
MgSeO4
12.8 Which Group 2 salts are used as drying
agents and why?
Anhydrous Mg, and Ca sulphates are
preferred as drying agents, because of the
higher affinity of Mg and Ca sulphates for
water.
12.9 How do group 2 salts give rise to scaling
from hard water?
Salts of divalent ions have low solubility.
12.10 Predict structures for BeTe and BaTe.
BeTe, close to ZnS-like structure. BaTe, close
to CsCl-like structure.
12.11 Use the data in Table 1.7 and the Ketelaar
triangle in Fig. 2.38 to predict the nature of
the bonding in BeBr2, MgBr2, and BaBr2.
BeBr2 should be covalent. MgBr2 should be
ionic. BaBr2 should be ionic
12.12 The two Grignard compounds C2H5MgBr
and 2,4,6-(CH3)3C6H2MgBr dissolve in THF.
What differences would be expected in the
structures of the species formed in these
solutions?
C2H5MgBr will be tetrahedral with two
molecules of solvent coordinated to the
magnesium. The bulky organic group in
2,4,6-(CH3)3C6 H2MgBr leads to a
coordination number of two.
12.13 Predict the products of the following
reactions?
(a) MgCl2 + 2LiC2H5 → 2LiCl +
Mg(C2H5)2
(b) Mg + (C2H5)2Hg → Mg(C2H5)2 + Hg
(c) Mg + C2H5HgCl → C2H5MgCl + Hg
CHAPTER 13
Self-tests
S13.1 11
B nuclei have I = 3/2. Predict the number
of lines and their relative intensities in the
1
H-NMR spectrum of BH4
–
? 4. Relative
intensity ratio is 1:3:3:1.
S13.2 Write an equation for the reaction of
LiBH4 with propene in ether solvent and a
1:1 stoichiometry and another equation for
its reaction with ammonium chloride in
THF with the same stoichiometry?
Simple alkenes are inert towards LiBH4.
LiBH4 + NH4Cl
THF
BH3NH3 + LiCl + H2
S13.3 Write and justify balanced equations for
plausible reactions between (a) BCl3 and
ethanol, (b) BCl3 and pyridine in
hydrocarbon solution, (c) BBr3 and
F3BN(CH3)3?
(a) BCl3 and ethanol?
BCl3(g) + 3 EtOH(l) → B(OEt)3(l) + 3 HCl(g)
(b) BCl3 and pyridine in hydrocarbon
solution?
BCl3(g) + py(l) → Cl3B − py(s)
(c) BBr3 and F3BN(CH3)3?
BBr3(l) + F3BN(CH3)3(s) → BF3(g) +
Br3BN(CH3)3(s)
S13.4 Suggest a reaction or series of reactions for
the preparation of N, N’, N’’-trimethyl-
B,B’,B’’-trimethylborazine starting with
methylamine and boron trichloride?
Cl3BNCH3 + 3CH3MgBr
(CH3)3B3N3(CH3)3 + 3Mg(Br, Cl)2
S13.5 How many framework electron pairs are
present in B4H10 and to what structural
category does it belong? Sketch its
structure?
7, arachno species.
The structure of B4H10:
S13.6 Propose a plausible product for the
reaction between Li[B10H13] and Al2(CH3)6?
[B10H11 (AlCH3)]–
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-20-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES 21
S13.7 Propose a synthesis for the polymer
precursor 1,7-B10C2H10(Si(CH3)2Cl)2 from 1,2-
B10C2H12 and other reagents of your choice?
1,2 - B10C2H12 1,7-B10C2H12 (90%) + 1,12-
B10C2H12 (10%)
⎯→
⎯Δ
1,7-B10C2H10Li2 + 2Si(CH3)2Cl2 → 1,7 -
B10C2H10(Si(CH3)2 + 2LiCl
S13.8 Propose, with reasons, the chemical
equation (or indicate no reaction) for
reactions between (a) (CH3)2SAlCl3 and
GaBr3?
(Me)2SalCl3 + GaBr3 → Me2SGaBr3 + AlCl3.
(b) TlCl and formaldehyde (HCHO) in
acidic aqueous solution? No reaction.
Exercises
13.1 Give a balanced chemical equation and
conditions for the recovery of boron?
B2O3 + 3Mg → 2B + 3MgO ΔH < 0
13.2 Describe the bonding in (a) BF3? Covalent.
(b) AlCl3? In the solid state, a layered
structure. At melting point, dimers. (c) B2H6?
Electron-deficient dimer.
13.3 Arrange the following in order of
increasing Lewis acidity: BF3, BCl3, AlCl3.
In the light of this order, write balanced
chemical reactions (or no reaction) for (a)
BF3N(CH3)3 + BCl3 →, (b) BH3CO +
BBr3→?
BCl3 > BF3 > AlCl3
(a) BF3N(CH3)3 + BCl3
BCl3N(CH3)3 + BF3 (BCl3 > BF3)
(b) BH3CO + BBr3 NR
13.4 Thallium tribromide (1.11 g) reacts
quantitatively with 0.257 g of NaBr to form
a product A. Deduce the formula of A.
Identify the cation and anion?
TlBr3 + NaBr → NaTlBr4
13.5 Identify compounds A, B, and C?
A
BF3
H2O
LiAlH4
B
C
heat
CaF2
(a) A = B2H6
(b) B = B(OH)3
rvive in air? If not, write the
2O3 + 3 H2O2
3.7 Predict how many different boron
13.8 products from the
(c) C = B2O3
13.6 Does B2H6 su
equation for the reaction?
No, it explodes in air.
B2H6 + 3 O2 → B
1
environments would be present in the
proton-decoupled 11
B-NMR of a) B5H11, b)
B4H10? a) 3 b) 2.
Predict the
hydroboration of (a) (CH3)2C=CH2, (b)
CH CH?
(a) H3 +
B (CH )2C=CH2
3
B[CH2-CH(CH3)2]3
(b) BH3 + CH CH
B(CH=CH2)3
Diborane ha
13.9 s been used as a rocket
extremely toxic, and the boron
3.10 Using BCl3 as a starting material and other
Æ B2Cl4 + 2HgCl2
3.11 Given NaBH4, a hydrocarbon of your
(a) BCl3 + 3C2H5MgCl
propellant. Calculate the energy released
from 1.00 kg of diborane given the
following values of ΔfHө
/kJ mol-1
: B2H6 =
31, H2O = -242, B2O3 = -1264. The
combustion reaction is B2H6 (g) + 3 O2(g)
→ 3 H2O (g) + B2O3 (s). What would be the
problem with diborane as a fuel?
-73,172 kJ.
Diborane is
containing product of combustion is a solid,
B2O3.
1
reagents of your choice, devise a synthesis
for the Lewis acid chelating agent, F2B–
C2H4–BF2?
2BCl3 + 2Hg
B2Cl4 + 4AgF Æ B2F4 + 4AgCl
B2F4 + C2H4 Æ F2CH2CH2BF2
1
choice, and appropriate ancillary reagents
and solvents, give formulas and conditions
for the synthesis of (a) B(C2H5)3, (b)
Et3NBH3?
Heat, Ether
B(C2H5)3 + 3MgCl2
(b) [HN(C2H5)3]Cl + NaBH4
H2 + H3BN(C2H5)3 + NaCl
3.12 Draw the B12 unit that is a common motif
1
of boron structures; take a viewpoint along
a C2 axis?
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-21-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES
22
13.13 Which boron hydride would you expect to
be more thermally stable, B6H10 or B6H12?
Give a generalization by which the thermal
stability of a borane can be judged?
B6H10
13.14 How many skeletal electrons are present in
B5H9? 14
13.15 (a) Give a balanced chemical equation
(including the state of each reactant and
product) for the air oxidation of
pentaborane(9). (b) Describe the probable
disadvantages, other than cost, for the use
of pentaborane as a fuel for an internal
combustion engine?
(a) 2B5H9 (l) + 12O2 (g)
Heat
5B2O3 (s) + 9H2O (l)
(b) The boron containing product of
combustion is a solid, B2O3.
13.16 (a) From its formula, classify B10H14 as
closo, nido, or arachno. (b) Use Wade’s
rules to determine the number of
framework electron pairs for
decaborane(14). (c) Verify by detailed
accounting of valence electrons that the
number of cluster valence electrons of
B10H14 is the same as that determined in
(b)?
(a) nido .
(b) 12.
(c) The total number of valence elections is
(10x3)+(14x1)=44; the number of cluster
valence is the remainder of 44-20=24.
13.17 Starting with B10H14 and other reagents of
your choice, give the equations for the
synthesis of [Fe(nido-B9C2H11)2]2-
, and
sketch the structure of this species?
(1) B10H14 + 2SEt2
B10H12(SEt2)2 + H2
(2) B10H12(SEt2)2 + C2H2
B10C2H12 + 2SEt2 + H2
(3) 2 B10C2H12 + 2EtO–
+ 4EtOH
2 B9C2H12
–
+ 2B(OEt)3 + 2H2
(4) Na[B9C2H12] + NaH
Na2[B9C2H11] + H2
(5) 2Na2[B9C2H11] + FeCl2
THF
2NaCl + Na2[Fe(B9C2H11)2]
B
B
B
B
C
C
B
B
B
B
B
Fe
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
B
B
B
B
C
C
B
B
B
B
B
2-
13.18 (a) What are the similarities and
differences in structure of layered BN and
graphite (Section 13.9)? (b) Contrast their
reactivity with Na and Br2. (c) Suggest a
rationalization for the differences in
structure and reactivity.
(a) Their structures? Both of these
substances have layered structures.
(b) Their reactivity with Na and
Br2? Graphite reacts, boron nitride is
unreactive.
(c) Explain the differences? The large
HOMO–LUMO gap in BN means it is more
difficult to remove an electron from it than
from the HOMO of graphite.
13.19 Devise a synthesis for the borazines (a)
Ph3N3B3Cl3 and (b) Me3N3B3H3, starting
with BCl3 and other reagents of your
choice. Draw the structures of the
products?
(a) Ph3N3B3Cl3?
3 PhNH3
+
Cl−
+ 3 BCl3 → Ph3N3B3Cl3 + 9 HCl
(b) Me3N3B3H3?
3 MeNH3
+
Cl−
+ 3 BCl3 → Me3N3B3Cl3 + 9 HCl
Me3N3B3Cl3 + 3 LiH → Me3N3B3H3 + 3 LiCl
The structures:
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-22-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES 23
13.20 Give the structural type and describe the
structures of B4H10, B5H9, and 1,2-
B10C2H12?
B4H10, is an arachno borane.
B5H9, is a nido borane.
1,2-B10C2H12 is a closo carborane
13.21 Arrange the following boron hydrides in
order of increasing Brønsted acidity, and
draw a structure for the probable structure
of the deprotonated form of one of them:
B2H6, B10H14, B5H9? In a series of boranes,
the acidity increases as the size of the borane
increases.
CHAPTER 14
Self-tests
S14.1 Describe how the electronic structure of
graphite is altered when it reacts with (a)
potassium, (b) bromine?
(a) With potassium? Potassium results in a
material with a higher conductivity.
(b) With bromine? Bromine can remove
electrons from the π-symmetry HOMOs of
graphite. This also results in a material with a
higher conductivity.
S14.2 Use the bond enthalpy data in Table 14.2
and above to calculate the standard
enthalpy of formation of CH4 and SiH4?
CH4: ΔfH= –61kJ mol–1
SiH4: ΔfH= +39 kJ mol–1
S14.3 Propose a synthesis of D13
CO2
–
starting
from 13
CO?
13
CO(g) + 2MnO2(s) → 13
CO2(g) + Mn2O3(s)
2Li(s) + D2 → 2LiD(s)
13
CO2(g) + LiD(et) → Li+
D13
CO2
−
(et)
Exercises
14.1 Silicon forms the chlorofluorides SiCl3F,
SiCl2F2, and SiClF3. Sketch the structures
of these molecules?
14.2 Explain why CH4 burns in air whereas CF4
does not. The enthalpy of combustion of
CH4 is −888 kJ mol−1
and the C–H and C–F
bond enthalpies are −413 and −489 kJ
mol−1
respectively?
The bond enthalpy of a C–F bond is higher
than the bond enthalpy of a C–H bond.
14.3 SiF4 reacts with (CH3)4NF to form
[(CH3)4N][SiF5]. (a) Use the VSEPR rules
to determine the shape of the cation and
anion in the product; (b) Account for the
fact that the 19
F NMR spectrum shows two
fluorine environments?
(a) The cation is [(CH3)4N]+
N
CH3
CH3
H3C
+
C
H3
The anion is SiF5
–
Si F
F
F
F
F
-
(b) There are two different fluorine
environments.
14.4 Draw the structure and determine the
charge on the cyclic anion [Si4O12]ν
?
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-23-320.jpg)

![ANSWERS TO SELF-TESTS AND EXERCISES 25
Calcium carbide contains discrete C2
2–
ions.
(c) K3C60? A solution of C60 can be treated
with elemental potassium. It is ionic.
14.15 Write balanced chemical equations for the
reactions of K2CO3 with HCl(aq) and of
Na4SiO4 with aqueous acid?
K2CO3(aq) + 2HCl(aq) → 2KCl(aq) + CO2(g) +
H2O(l)
Na4SiO4(aq) + 4HCl(aq) → 4NaCl(aq) + SiO2(s) +
2H2O(l)
14.16 Describe in general terms the nature of the
[SiO3]n
2ν
ion in jadeite and the silica-
alumina framework in kaolinite?
The structures of jadeite and kaolinite consist
of extended one- and two-dimensional
structures, respectively. The [SiO3
2–
]n ions in
jadeite are a linear polymer of SiO4
tetrahedra. The two-dimensional
aluminosilicate layers in kaolinite represent
another way of connecting SiO4 tetrahedra.
14.17 (a) How many bridging O atoms are in the
framework of a single sodalite cage? (b)
Describe the (supercage) polyhedron at the
centre of the Zeolite A structure in Fig.
14.3?
(a) 48.
(b) Eight sodalite cages are linked together to
form the large cage of zeolite A.
CHAPTER 15
Self-tests
S15.1 Consider the Lewis structure of a segment
of the structure of bismuth shown in Fig.
15.2. Is this puckered structure consistent
with the VSEPR model? Yes.
S15.2 Refined hydrocarbons and liquid hydrogen
are also used as rocket fuel. What are the
advantages of dimethylhydrazine over
these fuels?
Dimehylhydrazine ignites spontaneously, and
produces less CO2.
S15.3 From trends in the periodic table, decide
whether phosphorus or sulphur is likely to
be the stronger oxidizing agent? Sulphur.
S15.4 Summarize the reactions that are used for
the synthesis of hydrazine and
hydroxylamine. Are these reactions best
described as electron-transfer processes or
nucleophilic displacements?
NH3 + ClO−
+ H+
→ [H3N—Cl-O]−
+ H+
→
H2NCl + H2O
H2NCl + NH3 → H2NNH2 + HCl
Both can be thought of as redox reactions.
S15.5 When titrated against base a sample of
polyphosphate gave end points at 30.4 and
45.6 cm3
. What is the chain length?
There are (30.4 cm3
)/(7.6 cm3
) = 4 strongly
acidic OH groups per molecule. A molecule
with 2 terminal OH groups and four further
OH groups is a tetrapolyphosphate.
Exercises
15.1 List the elements in Groups 15 and indicate
the ones that are (a) diatomic gases, (b)
nonmetals, (c) metalloids, (d) true metals.
Indicate those elements that display the
inert-pair effect?
Type of
element
Diatomic
gas?
Inert pair
effect?
N Non-
metal
yes No
P Non-
metal
no No
As nonmetal no No
Sb metalloid no No
Bi metalloid no Yes
O nonmetal yes No
S nonmetal no No
Se nonmetal no No
Te nonmetal no No
15.2 (a) Give complete and balanced chemical
equations for each step in the synthesis of
H3PO4 from hydroxyapatite to yield (a)
high-purity phosphoric acid and (b)
fertilizer-grade phosphoric acid. (c)
Account for the large difference in costs
between these two methods?
(a) high-purity phosphoric acid?
2Ca3(PO4)2 + 10C + 6SiO2 →
P4
−
+ 10CO + 6CaSiO3
P4 (pure) + 5O2 → P4O10
P4O10 + 6H2O → 4H3PO4 (pure)
(b) Fertilizer grade H3PO4?
Ca5(PO4)3OH + 5H2SO4 →
3H3PO4 (impure) + 5CaSO4 + H2O
(c) Account for the difference in cost?
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-25-320.jpg)

![ANSWERS TO SELF-TESTS AND EXERCISES 27
15.12 Are reactions of NO2
–
as an oxidizing agent
generally faster or slower when pH is
lowered? Give a mechanistic explanation
for the pH dependence of NO2
–
oxidations?
The rates of reactions in which nitrite ion is
reduced are increased as the pH is lowered.
15.13 When equal volumes of nitric oxide (NO)
and air are mixed at atmospheric pressure
a rapid reaction occurs, to form NO2 and
N2O4. However, nitric oxide from an
automobile exhaust, which is present in the
parts per million concentration range,
reacts slowly with air. Give an explanation
for this observation in terms of the rate law
and the probable mechanism?
The rate law must be more than first order in
NO concentration.
15.14 Give balanced chemical equations for the
reactions of the following reagents with
PCl5 and indicate the structures of the
products: (a) water (1:1), (b) water in
excess, (c) AlCl3, (d) NH4Cl?
(a) H2O? tetrahedral POCl3.
PCl5 + H2O → POCl3 + 2HCl
(b) H2O in excess?
2PCl5(g) + 8H2O(l) → 2H3PO4(aq) +
10HCl(aq)
(c) AlCl3? tetrahedral.
PCl5 + AlCl3 → [PCl4]+
[AlCl4]−
(d) NH4Cl? Cyclic molecules or linear
chain polymers
nPCl5 + nNH4Cl →
−[(N = P(Cl)2)n]−
+ 4nHCl
15.15 Use standard potentials (Resource section
3) to calculate the standard potential of the
reaction of H3PO2 with Cu2+
. Are HPO2
2−
and H2PO2
2−
useful as oxidizing or
reducing agents?
Eº = 0.839 V.
HPO3
2–
and H2PO2
–
ions will be much better
reducing agents than oxidizing agents.
15.16 Identify the compounds A, B, C, and D?
A
AS B
C
D
Cl2
LiAlH4
Cl2/hv
3RMgBr
(a) A = AsCl3
(b) B = AsCl5
(c) C = AsR3
(d) D = AsH3
15.17 Sketch the two possible geometric isomers
of the octahedral [AsF4Cl2]–
and explain
how they could be distinguished by 19
F–
NMR?
The cis isomer gives two 19
F signals and the
trans isomer gives one signal.
15.18 Identify the nitrogen compounds A, B, C,
D, and E?
(a) A = NO2
(b) B = HNO3; C = NO
(c) D = N2O4
(d) E= NO2
(e) F = NH4
+
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-27-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES
28
1 Use the Latimer diagr
5.19 ams in Resource
R 16
ermine whether the decomposition of
ition of H2O2 is
ion of H2O2 is
–
S16.2 obable structures of SO2F and
with
xercis
e whether the following oxides are
mphoteric.
16.2 urce
section 3 to determine which species of N
and P disproportionate in acid conditions?
The species of N and P that disproportionate
+
are N2O4, NO, N2O, NH3OH , H4P2O6, and P.
CHAPTE
elf-tests
S
S16.1 Det
H2O2 is spontaneous in the presence of
either Br2 or Cl2?
The decompos
thermodynamically favored in presence of Br–
In Presence of Cl–
The decomposit
thermodynamically favored in presence of Cl
.
Pr -
(CH3) 3NSO2, and predict reactions
-
.
OH
Both are trigonal pyramidal. OH-
displaces
F— or (CH3) 3N—.
es
E
16.1 Stat
acidic, basic, neutral, or amphoteric: CO2,
P2O5, SO3, MgO, K2O, Al2O3, CO?
CO2, SO3, P2O5, and Al2O3 are a
CO is neutral; MgO and K2O are acidic.
(a) Use standard potentials (Reso
section 3) to calculate the standard
potential of the disproportionation of H2O2
in acid solution. (b) Is Cr2+
a likely catalyst
for the disproportionation of H2O2? (c)
Given the Latimer diagram
O2
HO2
-
-0.13 1.51
H2O2
in acidic solution, calculate Δr
ө
for the
of H2O2 and
Cr ? Cr is not capable
tion of HO2? ΔrG° =
157 kJ. For the disproportionation of H2O2
(part (a)), ΔrG° = 103 kJ.
16.3
tronger.
or SO2 (which
not react
16.5 pecies from the
agent: SO4 ,SO3 ,O3SO2SO3 ?
16.6
c
16.7 formula for Te(VI) in acidic
sible
ncrease its
16.8
ill disproportionate in
16.9
3 to predict whether
ble in acidic or basic
16.10 ether any of the following will be
: VO , Fe , Cu , Co ?
16.11 F4]. Use
hapes of the
cation and anion?
6.12 g)
nic
product. (a) Write a balanced equation for
G
disproportionation of hydrogen superoxide
(HO2–
)
into O2 and H2O2, and compare the
result with its value for the
disproportionation of H2O2?
(a) The disproportionation
HO2? 1.068 V.
(b) Catalysis by 2+ 2+
of decomposing H2O2.
(c) The disproportiona
Which hydrogen bond would be stronger:
S—H . . . O or O—H . . . S?
O–H hydrogen bonds are s
16.4 Which of the solvents ethylenediamine
(which is basic and reducing)
is acidic and oxidizing) might
with (a) Na2S4, (b) K2Te3?
ethylenediamine is a better solvent than sulfur
dioxide.
Rank the following s
strongest reducing agent to the strongest
oxidizing 2– 2– 2–
S2O8
2–
> SO4
2–
> SO3
2–
Predict which oxidation states of Mn will
be reduced by sulfite ions in basi
conditions?
Mn (+VII, +VI, +V, +IV, +III) will be
reduced by sulfite ions in basic solution.
(a) Give the
aqueous solution and contrast it with the
formula for S(VI). (b) Offer a plau
explanation for this difference?
(a) Formulas? H5TeO6
–
and HSO4
–
(b) An explanation? Tellurium is a larger
element than sulfur and can i
coordination number.
Use the standard potential data in
Resource section 3 to predict which
oxoanions of sulfur w
acidic conditions?
S2O6
2−
and S2O3
2−
.
Use the standard potential data in
Resource section
2–
SeO3 is more sta
solut on?
i
The SeO3
2–
is marginally more stable in acid
solutions.
Predict wh
reduced by thiosulfate ions,S2O3
2–
, in acidic
conditions 2+ 3+ + 3+
Fe3+
and Co3+
will be reduced.
SF4 reacts with BF3 to form [SF3][B
VSEPR theory to predict the s
SF4
+
trigonal pyramidal, BF4
−
tetrahedral.
1 Tetramethylammonium fluoride (0.70
reacts with SF4 (0.81 g) to form an io
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-28-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES 29
the reaction and (b) sketch the structure of
the anion. (c) How many lines would be
observed in the 19
F-NMR spectrum of the
anion?
(a) SF4 + (CH3)4NF → [(CH3)4N]+
[SF5]−
; (b)
square pyramidal structure; (c) two F
environments.
.13
of iodine is sodium iodate,
O3. Which of the reducing agents
SO (aq) or Sn2+
(aq) would seem practical
S17.2 r IF7?
and
several polyhalides that
to [py–I–py]+
, and describe
orbitals, one bonding, one
Exerci
17.1 reference
erial, write out the halogens and noble
y appear in the periodic table,
16 Identify the sulfur-containing compounds
A, B, C, D, E, and F?
A = S2Cl2, B = S4N4, C = S2N2, D = K2S2O3,
E = S2O6
2−
, F = SO2.
CHAPTER 17
Self-tests
S17.1 One source
NaI
2
from the standpoints of thermodynamic
feasibility and plausible judgements about
cost? Standard potentials are given in
Resource section 3?
Both. SO2 is cheaper.
Predict the 19
F-NMR pattern fo
2 resonances.
S17.3 From the perspective of structure
bonding, indicate
are analogous
their bonding?
Examples include I3
–
, IBr2
–
, ICl2
–
, and IF2
–
.
The three centers contribute four electrons to
three molecular
nonbonding, and one antibonding.
ses
Preferably without consulting
mat
gases as the
and indicate the trends in (a) physical state
(s, l, or g) at room temperature and
pressure, (b) electronegativity, (c) hardness
of the halide ion, (d) color?
Phy
sica
l
Electron
egativit
y
Har
dnes
s of
Col
or
stat
e
hali
de
ion
F
2
hest
(4.0)
light
yell
ow
gas hig hard
est
C
l2
gas lower soft
er
yell
ow-
gree
n
B
r2
liqu
id
lower soft
er
dark
red-
bro
wn
I2 soli
d
lowest soft
est
dark
viol
et
H
e
gas Col
orle
ss
N
e
gas colo
rless
A gas o
r
col
rless
K
r
gas colo
rless
X
e
gas colo
rless
17.2 Describe how the halogens are recovered
from their naturally occurring halides and
rationalize the approach in terms of
CaSO4 + 2 HF
2 KF → 2 K+
HF2
−
2 + 2 KF
Cl2 → X2 + 2 Cl−
(X−
= Br−
,
17.3 ow th
he dir
usion of the ions. Give the chemical
) → Cl2(g) + 2 e
cathod
H2O(l)
standard potentials. Give balanced
chemical equations and conditions where
appropriate?
For F:
F2 + H2SO4 →
2 HF +
2 K+
HF2
−
+ electricity → F2 + H
For Cl:
2 Cl−
+ 2 H2O + electricity → Cl2 + H2 +
2 OH−
For Br and I:
2 X−
+
I−
)
Sketch a choralkali cell. Sh e half-cell
reactions and indicate t ection of
diff
equation for the unwanted reaction that
would occur if OH–
migrated through the
membrane and into the anode
compartment?
A drawing of the cell is shown in Figure 17.3.
anode: 2 Cl−
(aq −
e: 2 H2O(l) + 2 e−
→ 2 OH−
(aq) + H2(g)
- unwanted reaction:
2 OH−
(aq) + Cl2(aq) → ClO−
(aq) +
Cl−
(aq) +
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-29-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES
30
17.4 Ske
a dihalogen ribe its role
tch the form of the vacant s* orbital of
molecule and desc
in the Lewis acidity of the dihalogens?
Since the 2σu* antibonding orbital is the
LUMO for a X2 molecule, it is the orbital that
17.5
le of oxidizing H2O to O2?
17.6 at –129˚C
void of Lewis basicity. By
.
effect in NF3
17.7 ogy between halogens
and pseudohalogens write: (a) the balanced
→ CN−
(aq) +
(b
aqueous aci
Mn (aq) + 2 H2O(l)
(c) l
cyan ins
17.8 3 reacts with 0.93 g of
[(CH3)4N]F to form a product X, (a) identify
X, (b) use the VSEPR model to predict the
accepts the pair of electrons from a Lewis
base.
Which dihalogens are thermodynamically
capab
Cl2 and F2.
Nitrogen trifluoride, NF3, boils
and is de
contrast, the lower molar mass compound
NH3 boils at –33˚C and is well known as a
Lewis base. (a) Describe the origins of this
very large difference in volatility. (b)
Describe the probable origins of the
difference in basicity?
(a) Difference in volatility? Ammonia
exhibits hydrogen bonding
(b) Explain the difference in basicity? The
strong electron-withdrawing
reduces the basicity.
Based on the anal
equation for the probable reaction of
cyanogen, (CN)2, with aqueous sodium
hydroxide, (b) the equation for the
probable reaction of excess thiocyanate
with the oxidizing agent MnO2(s) in acidic
aqueous solution, (c) a plausible structure
for trimethylsilyl cyanide?
(a) The reaction of NCCN with NaOH?
NCCN(aq) + 2 OH−
(aq)
NCO−
(aq) + H2O(l)
) The reaction of SCN–
with MnO2 in
d?
2SCN−
(aq) + MnO2(s) + 4 H+
(aq) →
(SCN)2(aq) + 2+
The structure of trimethylsily
ide? Trimethylsilyl cyanide conta
an Si–CN single bond.
Given that 1.84 g of IF
shapes of IF3 and the cation and anion in X,
(c) predict how many 19
F-NMR signals
would be observed in IF3 and X?
a). X = IF4N(CH3)4
b) Different possible arrangements of
IF3:
The shape of anion IF4 is Square planar.
The shape of cation (CH3)4N+
is
Tetrahedral.
c)
7.9 l to predict the shapes
5 3 ClF6]+
?
7.10
17.11 of the complexes
l3F3. Indicate how many
ents would be indicated
–
.IF3, two. IF4
–
, one.
1 Use the VSEPR mode
of SbCl, FClO , and [
SbCl5 is trigonal bipyramidal, FClO3 is
pyramidal, and ClF6 is octahedral.
1 Indicate the product of the reaction
between ClF5 and SbF5?
[ClF4]+
[SbF6] –
.
Sketch all the isomers
MCl4F2 and MC
fluorine environm
in the 19
F-NMR spectrum of each isomer?
MCl4F2, the cis isomer, 1; the trans isomer, 2.
MCl3F3, the fac isomer, 1 the mer isomer, 2.
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-30-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES 31
17.12 (a) Use the VSEPR model to predict the
probable shapes of [IF6]+
and IF7. (b) Give a
plausible chemical equation for the
preparation of [IF6][SbF6]?
(a) The structures of [IF6]+
and IF7? IF6
+
,
octahedral; IF7, pentagonal bipyramid.
(b) The preparation of [IF6] [SbF6]?
IF7 + SbF5 → [IF6][SbF6]
7.14
al, Cs
17.15 the following
s likely to make liquid BrF3 a
a stronger Lewis
he acidity or basicity
3
17.16 r
f IF5
+
? Two resonances.
17.17
xplain
) F2,
Since SbF5 cannot be oxidized, it
3.
reasoning?
2 IBr + Br−
17.19
not?
arge cations stabilize large, unstable anions.
17.13 Predict the shape of the doubly chlorine–
bridged I2Cl6 molecule by using the VSEPR
model and assign the point group?
A planar dimer (I2Cl6).
1 Predict the structure and identify the point
group of ClO F? trigonal pyramid
2
symmetry.
Predict whether each of
solutes i
stronger Lewis acid or
base: (a) SbF5, (b) SF6, (c) CsF?
(a) SbF5? increases the acidity of BrF3.
(b) SF6? No effect on t
of BrF .
(c) CsF? Increases the basicity of BrF3.
Predict the appea ance of the 19
F-NMR
spectrum o
.
Predict whether each of the following
compounds is likely to be dangerously
explosive in contact with BrF3 and e
your answer: (a) SbF5, (b) CH3OH, (c
(d) S2Cl2?
(a) SbF5?
will not form an explosive mixture with BrF
(b) CH3OH? Methanol, being an organic
compound, is readily oxidized by strong
oxidants.
(c) F2? No.
(d) S2Cl2? S2Cl2 will be oxidized to higher
valent sulfur fluorides.
17.18 The formation of Br3
–
from a
tetraalkylammonium bromide and Br2 is
only slightly exoergic. Write an equation
(or NR for no reaction) for the interaction
of [NR4][Br3] with I2 in CH2Cl2 solution and
give your
Br3
−
+ I2 →
Explain why CsI3(s) is stable with respect to
the elements but NaI3(s) is
L
17.20 Write plausible Lewis structures for (a)
ClO2 and (b) I2O6 and predict their shapes
and the associated point group?
(a).
(b) I2O5?
17.21 (a) Give the formulas and the probable
relative acidities of perbromic acid and
periodic acid. (b) Which is the more stable?
rmulas are HBrO4 and H5IO6. The
in iodine’s ability to expand its
coordination shell.
(b) Relative stabilities? Periodic acid is
thermodynamically more stable.
17.22 (a) Describe the expected trend in the
standard potential of an oxoanion in a
17.23
cission.
17.24 readily
chloric acid
a mechanistic
erence?
(a) The fo
difference lies
solution with decreasing pH. (b)
Demonstrate this phenomenon by
calculating the reduction potential of ClO4
–
at pH = 7 and comparing it with the
tabulated value at pH = 0?
(a) The expected trend? E decreases as the
pH increases.
(b) E at pH 0 and pH 7 for ClO4
–
?
Eº = 1.201 V (see Appendix 2).
At pH 7, V = 0.788 V
With regard to the general influence of pH
on the standard potentials of oxoanions,
explain why the disproportionation of an
oxoanion is often promoted by low pH?
Low pH results in a kinetic promotion:
protonation of an oxo group aids oxygen–
halogen bond s
Which oxidizing agent reacts more
in dilute aqueous solution, per
or periodic acid? Give
explanation for the diff
Periodic acid.
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-31-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES
32
17.25
lly
–
dard potentials.) (b)
7.26 following compounds present
4, (
losion hazard.
)
CHAP
f-tests
S18.1
e production of perxenate
O q OH−
(aq) → XeO6
4−
(aq) + Xe(g)
+ O2(g) + 2 H2O(l)
lain why helium is present in low
concentration in the atmosphere even
tive decay.
8.2 Which of the noble gases would you choose
(a) The lowest-temperature
igerant? Helium.
) XeF2? Xe and F2
ut have a large
) → XeF6(s)
18.4
(b) XeO2F2?
(a) For which of the following anions is
disproportionation thermodynamica
favourable in acidic solution: OCl2, ClO2 ,
ClO2
–
, and ClO4
–
? (If you do not know the
properties of these ions, determine them
from a table of stan
For which of the favourable cases is the
reaction very slow at room temperature?
The rates of disproportionation are probably
HClO > HClO2 > ClO3
–
.
1 Which of the
an explosion hazard? (a) NH4ClO4, (b)
Mg(ClO4)2, (c) NaClO d) [Fe(H2O)6][ClO4]2.
Explain your reasoning?
(a) NH4ClO4? Ammonium perchlorate is a
dangerous compound, since the N atom of the
NH4
+
ion is in its lowest oxidation state and
can be oxidized.
(b) Mg(ClO4)2? Not an explosion hazard.
(c) NaClO4? Not an exp
(d) [Fe(H2O)6] [ClO4]2? An explosion
hazard, since Fe(II) can be oxidized to Fe(III).
17.27 Use standard potentials to predict which of
the following will be oxidized by ClO–
ions
in acidic conditions: (a) Cr3+
, (b) V3+
, (c)
Fe2+
, (d) Co2+
?
(a) Cr3+
? No. (b) V3+
? Yes. (c) Fe2+
? Yes. (d
Co2+
? No.
TER 18
Sel
Write a balanced equation for the
decomposition of xenate ions in basic
solution for th
ions, xenon, and oxygen
4
−
(a ) + 2
2 HXe
xercises
E
18.1 Exp
though it is the second most abundant
element in the universe?
Helium present in today’s atmosphere is the
product of ongoing radioac
1
as
refr
(b) An electric discharge light source
requiring a safe gas with the lowest
ionization energy? Xenon.
(c) The least expensive inert
atmosphere? Argon.
18.3 By means of balanced chemical equations
and a statement of conditions, describe a
suitable synthesis of (a
at 400ºC, or photolyze Xe and F2 in glass:
Xe(g) + F2(g) → XeF2(s)
(b) XeF6? High temperature, b
excess of F2:
Xe(g) + 3 F2(g
(c) XeO3?
XeF6(s) + 3 H2O(l) → XeO3(s) + 6 HF(g)
Draw the Lewis structures of (a) XeOF4?
(c) XeO6
4−
?
18.5 ive the formula and describe the
structure of a noble gas species that is
isostructural with (a) ICl4
−
? XeF4
(b) IBr2
–
? Linear geometry. Isostructural
with XeF2.
(c) BrO3
–
? Trigonal pyramidal geometry.
Isostructural with XeO3.
(d) ClF? Isostructural with the cation XeF+
.
G
18.6 (a) Give a Lewis structure for XeF7
–
?
ith
bipyramid.
(b) Speculate on its possible structures by
using the VSEPR model and analogy w
other xenon fluoride anions? Pentagonal
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-32-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES 33
18.7 Use molecular orbital theory to calculate
the bond order of the diatomic species E2
+
with E=He and Ne? He2
2+
= 0.5, Ne2
2+
= 0.5.
18.8 Identify the xenon compounds A, , C, D,
and E?
4
other lines
b ted around the central
CHAP
19.1 Refer to the appropriate Latimer diagram
the
e and formula of the species
n?
19.2 Suggest a use for molybdenum(IV) sulfide
makes use of its solid-state structure.
onalize your suggestion? MoS2 used as
S19.3
ntaining PPh3?
olecular species such as Re3Cl12
3–
Exerci
19.1
es of the d block,
tion
ose for which
oup oxidation number is not achieved
by N?
B
A = XeF2(g)
B = [XeF]+
[MeBF3]
−
C = XeF6
D = XeO3
E = XeF (g).
18.9 Predict the appearance of the 129
Xe-NMR
spectrum of XeOF3
+
.
1:3:3:1 quartet
18.10 Predict the appearance of the 19
F-NMR
spectrum of XeOF4.
Strong c nt
e ral line, two
symmetrically distri u
line.
TER 19
Self-tests
S
in Resource section 3 and identify
oxidation stat
that is thermodynamically favoured when
an acidic aqueous solution of V2+
is exposed
to oxyge
+4; VO2
+
S
that
Rati
a lubricant. The slipperiness of MoS2 is
because of the ease with which one layer can
glide over another.
Describe the probable structure of the
compound formed when Re3Cl9 is dissolved
in a solvent co
Discrete m
or Re3Cl9(PPh3)3 are formed.
ses
Without reference to a periodic table,
sketch the first seri
including the symbols of the elements.
Indicate those elements for which the
group oxidation number is common by C,
those for which the group oxida
number can be reached but is a powerful
oxidizing agent by O, and th
the gr
19.2
As atoms become heavier, more energy is
needed to vaporize them.
19.3 State the trend in the stability of the group
oxidation state on descending a group of
metallic elements in the d block. Illustrate
the trend using standard potentials in
in
as you descend a group.
19.4
action) and
Fe2+
(aq)
(aq) →?
− 3+
1H2O →
19.5 q), is more
presence of
ith the
tren s
Pe al
More
Hardnes in the d
block.
19.6
e or fluorite
n of
example?
Explain why the enthalpy of sublimation of
Re(s) is significantly greater than that of
Mn(s)?
acidic solution for Groups 5 and 6.
The group oxidation number increases
stability
For each part, give balanced chemical
quations or NR (for no re
e
rationalize your answer in terms of trends
in oxidation states.
(a) Cr2+
(aq) + Fe3+
(aq) →?
Cr2+
(aq) + Fe3+
(aq) → Cr3+
(aq) +
(b) CrO4
2–
(aq) + MoO2 (s) →?
2 CrO4
2−
+ 3 MoO2(s) + 10 H+
→
2 Cr3+
+ 3 H2MoO4 + 2 H2O
(c) MnO4
–
(aq) + Cr3+
6 MnO4 + 10Cr + 1
6Mn2+
+ 5Cr2O7
2−
+ 22 H+
(a) Which ion, Ni2+
(aq) or Mn2+
(a
likely to form a sulfide in the
H2S? (b) Rationalize your answer w
ds in hard and soft character acros
riod 4. (c) Give a balanced chemic
equation for the reaction.
likely for Ni2+
to form a sulphide.
s decreases from left to right
Ni2+
(aq) + H2S(aq) → NiS(s) + 2 H+
(aq)
Preferably without reference to the text (a)
write out the d block of the periodic table,
(b) indicate the metals that form
difluorides with the rutil
structures, and (c) indicate the regio
the periodic table in which metal-metal
bonded halide compounds are formed,
giving one
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-33-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES
34
Metal–metal bonded halide compounds are
found within bold border. Example: Sc5Cl6.
19.7
ic
solution at +0.2 V is made strongly basic at
the same potential. Write a balanced
equation for each of the successive
reactions when this same complex at pH =
6 and +0.2 V is exposed to progressively
more oxidizing environments up to +1.0 V
–
tions
llowing
s for your
q) in
er an inert
O +
(a
(
Hg2+
(aq) + Cd(s) Cd2+
(aq)
19.9
r answers:
]
e cis-
o 4 , has a
an apical oxo
(b) significantly
19.11
ng
3)4]? σ2
π4
δ2
configuration,
2CC2H5)4]? σ2
π4
δ2
,chromium–
19.12 following
19.13 oate to aqueous
Write a balanced chemical equation for the
reaction that occurs when cis-
[RuLCl(OH2)]+
(see Fig. 19.9) in acid
.
Give other examples and a reason for the
redox state of the metal center affecting the
extent of protonation of coordinated
oxygen.
cis-[RuII
LCl(OH2)]+
+ Ox + OH−
→ cis-
[RuIII
LCl(OH)]+
+ Red + H2O
cis-[RuII
LCl(OH2)]+
+ H2O → cis-
[RuIII
LCl(OH)]+
+ H3O+
+ e−
cis-[RuIII
LCl(OH2)]+
+ H2O → cis-
[RuIV
LCl(OH)]+
+ H3O+
+ e−
As oxidation state of the metal increases,
ability to accept electron density from an OH
or O2–
ligand increases.
19.8 Give plausible balanced chemical reac
(or NR for no reaction) for the fo
combinations, and state the basi
answer: (a) MoO4
2–
(aq) plus Fe2+
(a
acidic solution? No reaction.
(b) The preparation of [Mo6O19]2−
(aq) from
K2MoO4(s)? 6 MoO4
2−
(aq) + 10 H+
(aq) →
[Mo6O19]2−
(aq) + 5 H2O(l)
(c) ReCl5 (s) plus KMnO4(aq)?
5 ReCl5(s) + 2 MnO4
−
(aq) + 12 H2O(l) →
5 ReO4
−
(aq) + 2 Mn2+
(aq) + 25 Cl−
(aq) +
24 H+
(aq)
(d) MoCl2(s) plus warm HBr(aq)?
6 MoCl2(s) + 2 Br−
(aq) → [Mo6Cl12]2−
(aq)
+ Br2(aq)
(e) TiO(s) with HCl(aq) und
atmosphere? 2Ti (s) + 6H q) Æ
2Ti3+
+ H2(g) + 2H2O(l) E˚ = +0.37 V.
f) Cd(s) added to Hg2+
(aq)?
Æ Hg(l) +
Speculate on the structures of the following
species and present bonding models to
justify you
(a) [Re(O)2 (py)4]+
, (b) [V (O)2 (ox)2]3–
, (c)
[Mo(O)2(CN)4
4–
, (d) [VOCl4]2–
? The first
three are dioxo complexes and will hav
metal structural units. (d) VOCl 2–
diox
square-pyramidal structure with
ligand.
19.10 Which of the following are likely to have
structures that are typical of (a)
predominantly ionic,
covalent, (c) metal-metal bonded
compounds: NiI2, NbCl4, FeF2, PtS, and
WCl2? Rationalize the differences and
speculate on the structures? (a) NiI2? ionic
compound with significant degree of covalent
character
(b) NbCl4? significantly covalent.
(c) FeF2? ionic.
(d) PtS ? significant amount of covalent
character.
(e) WCl2 ? metal–metal bonding.
Indicate the probable occupancy of s, p,
and d bonding and antibonding orbitals,
and the bond order for the followi
tetragonal prismatic complexes?
(a) [Mo2(O2CCH
molybdenum-molybdenum quadruple bond.
(b) [Cr2(O
chromium quadruple bond.
(c) [Cu2(O2CCH3)4]? σ2
π4
δ2
δ*2
π*4
σ*2
configuration, no metal–metal bond in this
molecule.
Explain the differences in the
redox couples, measured at 25o
C?
Higher oxidation states become more stable
.
on descending a group
Addition of sodium ethan
solutions of Cr(II) gives a red diamagnetic
product. Draw the structure of the
product, noting any features of interest?
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-34-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES 35
Cr O
O
O
O O
O
O
Cr
O
19.14 Consider the two ruthenium complexes in
Table 19.8. Using the bonding scheme
depicted in Figure 19.19, confirm the
bonding orders and electron configurations
given in the table.
[Ru2Cl2(ClCO2)4]-
:
2.5 +, mixed valence at the Ru center, in line
with the energy level scheme [0.5 (8-3)].
[Ru2 (CH3COCH3)2(ClCO2)4]: 2+, the bond
order of 2.0 should also be expected [0.5x(8-
4)].
CHAPTER 20
Self-tests
S20.1 What is the LFSE for both high- and low-
spin d7
configurations? A high-spin d7
configuration is t2
5
geg
2
. High spin, 0.8 Δo.
S20.2 The magnetic moment of the complex
[Mn(NCS)6]4–
is 6.06μB. What is its electron
configuration? t2g
3
eg
2
S20.3 Account for the variation in lattice
enthalpy of the solid fluorides in which
each metal ion is surrounded by an
octahedral array of F–
ions: MnF–
(2780 kJ
mol–1
), FeF2 (2926 kJ mol–1
), CoF2 (2976 kJ
mol–1
), NiF2 (3060 kJ mol–1
), and ZnF2
(2985 kJ mol–1
).
If it were not for ligand field stabilization
energy (LFSE), MF2 lattice enthalpies would
increase from Mn(II) to Zn(II).
S20.4 Suggest an interpretation of the
photoelectron spectra of [Fe(C5H5)2] and
[Mg(C5H5)2] shown in Fig. 20.18?
In the spectrum of [Mo(CO)6], the ionization
energy around 8 eV was attributed to the t2g
electrons that are largely metal-based. The
differences in the 6–8 eV region can be
attributed to the lack of d electrons for Mg(II).
S20.5 What terms arise from a p1
d1
configuration? 1
F and 3
F terms are possible.
S20.6 Identifying ground terms (Hint: Because d9
is one electron short of a closed shell with L
= 0 and S = 0, treat it on the same footing
as a d1
configuration.) (a) 2p2
? 3
P (called a
“triplet P” term).
(b) 3d9
? 2
D (called a “doublet D” term).
S20.7 What terms in a d2
complex of Oh
symmetry correlate with the 3
F and 1
D
terms of the free atom? An F terms are
3
T1g, 3
T2g, and 3
A2g. Similarly, D terms are
1
T2g and 1
Eg.
S20.8 Use the same Tanabe-Sugano diagram to
predict the energy of the first two spin-
allowed quartet bands in the spectrum of
[Cr(OH2)6]3+
for which Δo = 17 600 cm-1
and B = 700 cm-1
.
17500 cm–1
and 22400 cm–1
.
S20.9 The spectrum of [Cr(NCS)6]3-
has a very
weak band near 16 000 cm-1
, a band at 17
700 cm-1
with εmax= 5160 dm3
mol-1
cm-1
, a
band at 23 800 cm-1
with εmax= 5130 dm3
mol-1
cm-1
, and a very strong band at 32
400 cm-1.
Assign these transitions using the
d3
Tanabe-Sugano diagram and selection
rule considerations. (Hint: NCS-
has low-
lying π* orbitals.)
(i) The very low intensity of the band at 16,000
cm–1
is a clue that it is a spin-forbidden
transition, probably 2
Eg ← 4
A2g.
(ii) Spin-allowed but Laporte-forbidden bands
typically have ε ~ 100 M–1
cm–1
, so it is
likely that the bands at 17,700 cm–1
and
23,800 cm–1
are of this type.
(iii) The band at 32,400 cm–1
is probably a charge
transfer band, since its intensity is too
high to be a ligand field (d–d) band.
Exercises
20.1 Determine the configuration (in the form
t2g
x
eg
y
or ex
t2
y
, as appropriate), the
number of unpaired electrons, and the
ligand-field stabilization energy in terms of
ΔO or ΔT and P for each of the following
complexes using the spectrochemical series
to decide, where relevant, which are likely
to be high-spin and which low-spin.
(a) [Co(NH3)6]3+
? d6
, low spin, no unpaired
electrons, LFSE = 2.4Δ0.
(b) [Fe(OH2)6]2+
? d6
, high spin, four
unpaired electrons, LFSE = 0.4Δ0.
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-35-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES
36
(c) [Fe(CN)6]3–
? d5
, low spin, one unpaired
electron. LFSE is 2.0Δ0.
(d) [Cr(NH3)6]3+
? d3
, three unpaired
electrons, low spin, LFSE = 1.2Δ0.
(e) [W(CO)6]? d6
, low spin, no unpaired
electrons, LFSE = 2.4Δ0.
(f) Tetrahedral [FeCl4]2−
? d6
, high spin,
four unpaired electrons, LFSE = 0.6 ΔT.
(g) Tetrahedral [Ni(CO)4]? d10
, 0 unpaired
electrons, LFSE = 0.
20.2 Both H-
and P(C6H5)3 are ligands of similar
field strength, high in the spectrochemical
series. Recalling that phosphines act as π
acceptors, is π-acceptor character required
for strong-field behaviour? What orbital
factors account for the strength of each
ligand?
No.
Thus there are two ways for a complex to
develop a large value of Δ0, by possessing
ligands that are π-acids or by possessing
ligands that are strong σ-bases (or both).
20.3 Estimate the spin-only contribution to the
magnetic moment for each complex in
Exercise 20.1. Spin-only contributions are:
complex N μSO = [(N)(N + 2)]1/2
[Co(NH3)6]3+
0 0
[Fe(OH2)6]2+
4 4.9
[Fe(CN)6]3–
1 1.7
[Cr(NH3)6]3+
3 3.9
[W(CO)6] 0 0
[FeCl4]2–
4 4.9
[Ni(CO)4] 0 0
20.4 Solutions of the complexes [Co(NH3)6]2+
,
[Co(OH2)6]2+
(both Oh), and [CoCl4]2-
are
colored. One is pink, another is yellow, and
the third is blue. Considering the
spectrochemical series and the relative
magnitudes of ΔT and ΔO, assign each color
to one of the complexes. [CoCl4]2–
is blue,
[Co(NH3)6]2+
is yellow, [Co(OH2)6]2+
is pink.
20.5 For each of the following pairs of
complexes, identify the one that has the
larger LFSE:
(a) [Cr(OH2)6]2+
or [Mn(OH2)6]2+
(b) [Mn(OH2)6]2+
or [Fe(OH2)6]3+
(c) [Fe(OH2)6]3+
or [Fe(CN)6]3-
(d) [Fe(CN)6]3–
or [Ru(CN)6]
(e) tetrahedral [FeCl4]2–
or tetrahedral
[CoCl4]2–
(a) The chromium complex.
(b) The Fe3+
complex.
(c) [Fe(CN)6]3–
(d) The ruthenium complex.
(e) The Co2+
complex.
20.6 Interpret the variation, including the overall
trend across the 3d series, of the following
values of oxide lattice enthalpies (in kJ
mol–1
). All the compounds have the rock-
salt structure: CaO (3460), TiO (3878), VO
(3913), MnO (3810), FeO (3921), CoO
(3988), NiO (4071)? There are two factors
that lead to the values: (i) decreasing ionic
radius from left to right across the d block,
and (ii) LFSE.
.20.7 A neutral macrocyclic ligand with four
donor atoms produces a red diamagnetic
low-spin d8
complex of Ni(II) if the anion is
the weakly coordinating perchlorate ion.
When perchlorate is replaced by two
thiocyanate ions, SCN–
, the complex turns
violet and is high-spin with two unpaired
electrons. Interpret the change in terms of
structure? Shift from square planar to
tetragonal complex.
20.8 Bearing in mind the Jahn-Teller theorem,
Predict the structure of
[Cr(OH2)6]2+
? Elongated octahedron.
20.9 The spectrum of d1
Ti3+
(aq) is attributed to
a single electronic transition eg ← t2g. The
band shown in Fig. 20.3 is not symmetrical
and suggests that more than one state is
involved. Suggest how to explain this
observation using the Jahn-Teller
theorem? The electronic excited state of
Ti(OH2)6
3+
has the configuration t2g
0
eg
1
, and
so the excited state possesses eg degeneracy.
Therefore, the “single” electronic transition is
really the superposition of two transitions, one
from an Oh ground-state ion to an Oh excited-
state ion, and a lower energy transition from
an Oh ground-state ion to a lower energy
distorted excited-state ion.
20.10 Write the Russell–Saunders term symbols
for states with the angular momentum
quantum numbers (L,S):
(a) L = 0, S = 5/2? 6
S
(b) L = 3, S = 3/2? 4
F
(c) L = 2, S = 1/2? 2
D
(d) L = 1, S = 1? 3
P
20.11 Identify the ground term from each set of
terms: (a) 3
F, 3
P, 1
P, 1
G? 3
F.
(b) 5
D, 3
H, 3
P, 1
G, 1
I? 5
D
(c) 6
S, 4
G, 4
P, 2
I? 6
S
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-36-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES 37
20.12 Give the Russell-Saunders terms of the
configurations and identify the ground
term? (a) 4 s1
? 2
S.
(b) 3p2
? 1
D, 3
P, 1
S. The ground term is 3
P.
20.13 The gas-phase ion V3+
has a 3
F ground term.
The 1
D and 3
P terms lie, respectively, 10
642 and 12 920 cm–1
above it. The energies
of the terms are given in terms of Racah
parameters as E(3
F) = A - 8B, E(3
P) = A +
7B, E(1
D) = A - 3B + 2C. Calculate the
values of B and C for V3+
. B = (12920 cm–
1
)/(15) = 861.33 cm–1
and C = 3167.7 cm–1
.
20.14 Write the d-orbital configurations and use
the Tanabe–Sugano diagrams (Resource
section 6) to identify the ground term of (a)
Low-spin [Rh(NH3)6]3+
? 1
A1g.
(b) [Ti(H2O)6]3+
? 2
T2g.
(c) High-spin [Fe(H2O)6]3+
? 6
A1g.
20.15 Using the Tanabe-Sugano diagrams in
Resource section 6, estimate ΔO and B for
(a) [Ni(H2O)6]2+
(absorptions at 8500, 15400
and 26000 cm-1
) ? Δ0 = 8500 cm–1
and B ≈
770 cm–1
.
(b) [Ni(NH3)6]2+
(absorptions at 10750,
17500 and 28200 cm-1
)? Δ0 = 10,750 cm–1
and B ≈ 720 cm–1
.
20.16 The spectrum of [Co(NH3)6]3+
has a very
weak band in the red and two moderate
intensity bands in the visible to near-UV.
How should these transitions be assigned?
The first two transitions listed above
correspond to two low-spin bands. The very
weak band in the red corresponds to a spin-
forbidden transition.
20.17 Explain why [FeF6]3-
is colorless whereas
[CoF6]3–
is colored but exhibits only a
single band in the visible. No spin-allowed
transitions are possible for the Fe3+
; the
complex is expected to be colorless. The d6
Co3+
ion in [CoF6]3–
is also high spin, but in
this case a single spin-allowed transition
makes the complex colored and gives it a one-
band spectrum.
20.18 The Racah parameter B is 460 cm–1
in
[Co(CN)6]3-
and 615 cm-1
in [Co(NH3)6]3+
.
Consider the nature of bonding with the
two ligands and explain the difference in
nephelauxetic effect? Ammonia and cyanide
ion are both σ-bases, but cyanide is also a π-
acid.
20.19 An approximately ‘octahedral’ complex of
Co(III) with ammine and chloro ligands
gives two bands with εmax between 60 and
80 dm3
mol–1
cm–1
, one weak peak with
εmax52 dm3
mol-1
cm-1
, and a strong band at
higher energy with εmax= 2 x 104
dm3
mol–1
cm–1
. What do you suggest for the origins
of these transitions? First, the intense band
at relatively high energy is undoubtedly a
spin-allowed charge-transfer transition. The
two bands with εmax = 60 and 80 M–1
cm–1
are
probably spin-allowed ligand field transitions.
The very weak peak is most likely a spin-
forbidden ligand field transition.
20.20 Ordinary bottle glass appears nearly
colorless when viewed through the wall of
the bottle but green when viewed from the
end so that the light has a long path
through the glass. The color is associated
with the presence of Fe3+
in the silicate
matrix. Suggest which transitions are
responsible for the color? The faint green
color, which is only observed when looking
through a long pathlength of bottle glass, is
caused by spin-forbidden ligand field
transitions.
20.21 Solutions of [Cr(OH2)6]3+
ions are pale blue–
green but the chromate ion, CrO4
2–
, is an
intense yellow. Characterize the origins of
the transitions and explain the relative
intensities. The blue-green color of the Cr3+
ions in [Cr(H2O)6]3+
is caused by spin-
allowed but Laporte-forbidden ligand field
transitions. The relatively low molar
absorption coefficient is the reason that the
intensity of the color is weak. The oxidation
state of chromium in dichromate dianion is
Cr(VI); the intense yellow color is due to
LMCT transitions.
20.22 Classify the symmetry type of the d orbital
in a tetragonal C4v symmetry complex, such
as [CoCl(NH3)5] –
, where the Cl lies on the
z-axis. (a) Which orbitals will be displaced
from their position in the octahedral
molecular orbital diagram by π
interactions with the lone pairs of the Cl–
ligand? (b) Which orbital will move
because the Cl–
ligand is not as strong a
base as NH3? (c) Sketch the qualitative
molecular orbital diagram for the C4v
complex. The Cl atom lone pairs of electrons
can form π molecular orbitals with dxz and dyz.
These metal atomic orbitals are π-antibonding
MOs, and so they will be raised in energy.
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-37-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES
38
20.23 Consider the molecular orbital diagram for
a tetrahedral complex (based on Fig. 20.7)
and the relevant d-orbital configuration
and show that the purple color of MnO4
-
ions cannot arise from a ligand-field
transition. Given that the wavenumbers of
the two transitions in MnO4
-
are 18 500 and
32 200 cm–1
, explain how to estimate ΔT
from an assignment of the two charge-
transfer transitions, even though ΔT cannot
be observed directly. The oxidation state of
manganese in permanganate anion is
Mn(VII), which is d0
. Therefore, no ligand
field transitions are possible.
The difference in energy between the two
transitions, E(t2) – E(e) = 13700 cm–1
, is just
equal to ΔT.
20.24 The lowest energy band in the spectrum of
[Fe(OH2)6]3+
(in 1M HClO4) occurs at
lower energy than the equivalent transition
in the spectrum of [Mn(OH2)6]2+
. Explain
why this is.
The extra charge of the iron complexes keeps
the eg and t2g levels close
CHAPTER 21
Self-tests
S21.1 Calculate the second-order rate constant
for the reaction of trans-[PtCl(CH3)
(PEt3)2] with NO2
–
in MeOH, for which npt
= 3.22 ? k2(NO2
−
) = 100.71
= 5.1 M−1
s−1
S21.2 Given the reactants PPh3, NH3, and [PtCl4]2-
,
Propose efficient routes to both cis- and
trans- [PtCl2(NH3)(PPh3)]?
S21.3 Use the data in Table 21.8 to estimate an
appropriate value for KE and calculate kr2
for the reactions of V(II) with Cl-
if the
observed second-order rate constant is
1.2x102
dm3
mol–1
s–1
. KE = 1 M–1
, k = 1.2 ×
102
s–1
.
Exercises
21.1 The rate constants for the formation of
[CoX(NH3)5]2+
from [Co(NH3)5OH2]3+
for X
= Cl2, Br2, N3
–
, and SCN¯ differ by no more
than a factor of two. What is the
mechanism of the substitution? Dissociative.
21.2 If a substitution process is associative, why
may it be difficult to characterize an aqua
ion as labile or inert? The rate of an
associative process depends on the identity of
the entering ligand and, therefore, it is not an
inherent property of [M(OH2)6]n+
.
21.3 The reactions of Ni(CO)4 in which
phosphines or phosphites replace CO to
give Ni(CO)3L all occur at the same rate
regardless of which phosphine or phosphite
is being used. Is the reaction d or a? d.
21.4 Write the rate law for formation of
[MnX(OH2)5]1 from the aqua ion and X–
.
How would you undertake to determine if
the reaction is d or a?
rate = (kKE[Mn(OH2)6
2+
][X−
])/(1 + KE[X−
])
21.5 Octahedral complexes of metal centers with
high oxidation numbers or of d metals of
the second and third series are less labile
than those of low oxidation number and d
metals of the first series of the block.
Account for this observation on the basis of
a dissociative rate-determining step.
Metal centers with high oxidation numbers
have stronger bonds to ligands than metal
centers with low oxidation numbers.
Furthermore, period 5 and 6 d-block metals
have stronger metal ligand bonds.
21.6 A Pt(II) complex of
tetramethyldiethylenetriamine is attacked
by Cl-
105
times less rapidly than the
diethylenetriamine analogue. Explain this
observation in terms of an associative rate-
determining step. The greater steric
hindrance.
21.7 The rate of loss of chlorobenzene, PhCl,
from [W(CO)4L(PhCl)] increases with
increase in the cone angle of L. What does
this observation suggest about the
mechanism? The mechanism is
dissociative.
21.8 The pressure dependence of the
replacement of chlorobenzene (PhCl) by
piperidine in the complex
[W(CO)4(PPh3)(PhCl)] has been studied.
The volume of activation is found to be
111.3 cm3
mol–1
. What does this value
suggest about the mechanism? Mechanism
must be dissociative.
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-38-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES 39
21.9 Does the fact that [Ni(CN)5]3–
can be
isolated help to explain why substitution
reactions of [Ni(CN)4]2–
are very rapid?
For a detectable amount of [Ni(CN)5]3–
to
build up in solution, the forward rate constant
kf must be numerically close to or greater than
the reverse rate constant kr.
21.10 Reactions of [Pt(Ph)2(SMe2)2] with the
bidentate ligand 1,10-phenanthroline
(phen) give [Pt(Ph)2phen]. There is a
kinetic pathway with activation
parameters Δ‡
H = 1101 kJ mol–1
and Δ‡
S
= 142 J K–1
mol–1
. Propose a mechanism.
A possible mechanism is loss of one
dimethyl sulfide ligand, followed by the
coordination of 1,10-phenanthroline.
21.11 A two-step synthesis for cis- and trans-
[PtCl2(NO2) (NH3)] –
start with [PtCl4]2–
?
21.12 How does each of the following affect the
rate of square-planar substitution
reactions? (a) Changing a trans ligand
from H to Cl? Rate decreases.
(b) Changing the leaving group from Cl–
to
I–
? The rate decreases.
(c) Adding a bulky substituent to a cis
ligand? The rate decreases.
(d) Increasing the positive charge on the
complex? Rate increase.
21.13 The rate of attack on Co(III) by an entering
group Y is nearly independent of Y with
the spectacular exception of the rapid
reaction with OH–
. Explain the anomaly.
What is the implication of your explanation
for the behaviour of a complex lacking
Brønsted acidity on the ligands? (i) The
general trend: octahedral Co(III) complexes
undergo dissociatively activated ligand
substitution.
(ii) The anomalously high rate of substitution
by OH–
signals an alternate path, that of base
hydrolysis.
(iii) The implication is that a complex without
protic ligands will not undergo anomalously
fast OH–
ion substitution.
21.14 Predict the products of the following
reactions: (a) [Pt(PR3)4]2+
+ 2Cl–
? cis-
[PtCl2(PR3)2].
(b) [PtCl4]2–
+ 2PR3? trans-[PtCl2(PR3)2].
(c) cis-[Pt(NH3)2(py)2]2+
+ 2Cl–
? trans-
[PtCl2(NH3)(py)].
21.15 Put in order of increasing rate of
substitution by H2O the complexes:
[Co(NH3)6]3+
, [Rh(NH3)6]3+
, [Ir(NH3)6]3+
,
[Mn(OH2)6]2+
, [Ni(OH2)6]2+
?
The order of increasing rate is [Ir(NH3)6]3+
<
[Rh(NH3)6]3+
< [Co(NH3)6]3+
< [Ni(OH2)6]2+
< [Mn(OH2)6]2+
.
21.16 State the effect on the rate of dissociatively
activated reactions of Rh(III) complexes of
each of (a) an increase in the positive
charge on the complex? decreased rate.
(b) changing the leaving group from NO3
–
to Cl–
? Decreased rate.
(c) changing the entering group from Cl–
to
I–
? This change will have little or no effect
on the rate.
(d) changing the cis ligands from NH3 to
H2O? Decreased rate.
21.17 Write out the inner- and outer-sphere
pathways for reduction of
azidopentaamminecobalt(III) ion with
V2+
(aq). What experimental data might be
used to distinguish between the two
pathways?
The inner-sphere pathway:
[Co(N3)(NH3)5]2+
+ [V(OH2)6]2+
→
[[Co(N3)(NH3)5]2+
, [V(OH2)6]2+
}
{[Co(N3)(NH3)5]2+
, [V(OH2)6]2+
} →
{[Co(N3)(NH3)5]2+
, [V(OH2)5]2+
, H2O}
{[Co(N3)(NH3)5]2+
, [V(OH2)5]2+
, H2O} →
[(NH3)5Co–N=N=N–V(OH2)5]4+
[(NH3)5Co–N=N=N–V(OH2)5]4+
→ [(NH3)5Co–
N=N=N–V(OH2)5]4+
[(NH3)5Co–N=N=N–V(OH2)5]4+
→ [Co(OH2)6]2+
+
[V(N3)(OH2)5]2+
The outer sphere pathway:
[Co(N3)(NH3)5]2+
+ [V(OH2)6]2+
→
{[Co(N3)(NH3)5]2+
[V(OH2)6]2+
}
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-39-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES
40
{[Co(N3)(NH3)5]2+
,[V(OH2)6]2+
} → {[Co(N3)(NH3)5]+
,
[V(OH2)6]3+
}
{[Co(N3)(NH3)5] +
,[V(OH2)6]3+
} → {[Co(OH2)6]2+
,
[V(OH2)6]3+
21.18 The compound [Fe(SCN)(OH2)5]2+
can be
detected in the reaction of
[Co(NCS)(NH3)5]2+
with Fe2+
(aq) to give
Fe3+
(aq) and Co2+
(aq). What does this
observation suggest about the
mechanism? Appears to be an inner-sphere
electron transfer reaction.
21.19 Calculate the rate constants for electron
transfer in the oxidation of [V(OH2)6]2+
(Eσ
(V3+
/V2+
) = –0.255 V) and the oxidants (a)
[Ru(NH3)6]3+
(EO
(Ru3+
/Ru2+
) = + 0.07 V),
(b) [Co(NH3)6]3+
(EO
(Co3+
/Co2+
) = +0.10 V).
Comment on the relative sizes of the rate
constants.
(a) [Ru(NH3)6]3+
k = 4.53 × 103
dm3
mol−1
s−1
(b) [Co(NH3)6]3+
k = 1.41 × 10−2
dm3
mol−1
s−1
Relative sizes? The reduction of the Ru
complex is more thermodynamically favoured
and faster.
21.20 Calculate the rate constants for electron
transfer in the oxidation of [Cr(OH2)6]2+
(EO
–(Cr3+
/Cr2+
) = –0.41 V) and each of the
oxidants [Ru(NH3)6]3+
(EO
(Ru3+
/Ru2+
) =
+0.07 V), [Fe(OH2)6]3+
(EO
(Fe3+
/Fe2+
) =
+0.77 V) and [Ru(bpy)3]3+
(EO
(Ru3+
/Ru2+
) =
+1.26 V). Comment on the relative sizes of
the rate constants
(a) k11 (Cr3+
/Cr2+
) = 1 × 10−5
dm3
mol−1
s−1
; k22
(Ru3+
/Ru2+
for the hexamine complex) = 6.6 ×
103
dm3
mol−1
s−1
; f12 = 1; K12 = e [nF εo/RT]
where εo
= 0.07 V – (–0.41V) = 0.48 V; n = 1;
F = 96485 C; R = 8.31 Jmol−1
K−1
and T = 298
K. Using these values gives K12 = 1.32 × 108
.
Substitution of these values in the Marcus-
Cross relationship gives k12 = 2.95 × 103
dm3
mol−1
s−1
.
(b) k11 (Cr3+
/Cr2+
) = 1 × 10−5
dm3
mol−1
s−1
; k22
(Fe3+
/Fe2+
for the aqua complex) = 1.1
dm3
mol−1
s−1
; f12 = 1; K12 = e[nF εo/RT]
where εo
= 0.77 V – (−0.41V) = 1.18 V; n = 1; F =
96485 C; R = 8.31 Jmol−1
K−1
and T = 298 K.
Using these values we get K12 = 9.26 × 1019
.
Substitution of these values in the Marcus-
Cross relationship gives k12 = 3.19 × 107
dm3
mol−1
s−1
.
(c) k11 (Cr3+
/Cr2+
) = 1 × 10−5
dm3
mol−1
s−1
;
k22 (Ru3+
/Ru2+
for the bipy complex) = 4 × 108
dm3
mol−1
s−1
; f12 = 1; K12 = e[nF εo/RT]
where εo
= 1.26 V – (–0.41V) = 1.67 V; n = 1; F =
96485 C; R = 8.31 Jmol−1
K−1
and T = 298 K.
Using these values gives K12 = 1.81 × 1028
.
Substitution of these values in the Marcus-
Cross relationship gives k12 = 8.51 × 1015
dm3
mol−1
s−1
.21.21 The
photochemical substitution of
[W(CO)5(py)] (py = pyridine) with
triphenylphosphine gives
W(CO)5(P(C6H5)3). In the presence of
excess phosphine, the quantum yield is
approximately 0.4. A flash photolysis study
reveals a spectrum that can be assigned to
the intermediate W(CO)5. What product
and quantum yield do you predict for
substitution of [W(CO)5(py)] in the
presence of excess triethylamine? Is this
reaction expected to be initiated from the
ligand field or MLCT excited state of the
complex? The product will be
[W(CO)5(NEt3)], and the quantum yield will
be 0.4.
21.22 From the spectrum of [CrCl(NH3)s]2+
shown
in Fig. 20.32, propose a wavelength for
photoinitiation of reduction of Cr(III) to
Cr(II) accompanied by oxidation of a
ligand. ~250 nm.
CHAPTER 22
Self-tests
S22.1 Is Mo(CO)7 likely to be stable? No.
S22.2 What is the electron count for and
oxidation number of platinum in the anion
of Zeise’s salt, [PtCl3(C2H4)]–
? Treat
CH2=CH2 as a neutral two-electron donor.
Electron count, 16; oxidation number, +4.
S22.3 What is the formal name of
[Ir(Br)2(CH3)(CO)(PPh3)2]?
Dibromocarbonylmethylbis(triphenylphos-
phine)iridium(III).
S22.4 Which of the two iron compounds Fe(CO)5
and [Fe(CO)4(PEt3)] will have the higher
CO stretching frequency? Which will have
the longer M–C bond? Fe(CO)5
S22.5 Show that both are 18-electron species.
(a) [(η6
-C7H8)Mo(CO)3] (49)?
η6
-C7H8 = 6 electrons, Mo atom = 6.
carbonyl = 2 electrons, total = 18.
(b) [(η7
-C7H7)Mo(CO)3]+
(51)? η7
-C7H7
+
= 6
electrons, Mo atom = 6, 3 COs = 6, total = 18.
S22.6 Propose a synthesis for
Mn(CO)4(PPh3)(COCH3) starting with
[Mn2(CO)10], PPh3, Na and CH3I.
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-40-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES 41
Mn2(CO)10 + 2 Na → 2Na[Mn(CO)5]
Na[Mn(CO)5] + CH3I → Mn(CH3)(CO)5 + NaI
Mn(CH3)(CO)5 + PPh3 → Mn(CO)4(PPh3)(COCH3)
S22.7 The IR spectrum of [Ni2(η5-Cp)2(CO)2] has
a pair of CO stretching bands at 1857 cm–1
(strong) and 1897 cm–1
(weak). Does this
complex contain bridging or terminal CO
ligands, or both? (Substitution of η5-C5H5
ligands for CO ligands leads to small shifts
in the CO stretching frequencies for a
terminal CO ligand.) Bridging
S22.8 By using the same molecular orbital
diagram, comment on whether the removal
of an electron from [Fe(η5-Cp)2] to
produce [Fe(η5-Cp)2]+
should produce a
substantial change in M–C bond length
relative to neutral ferrocene. It will not.
S 22.9 The compound [Fe4(Cp)4(CO)4] is a dark-
green solid. Its IR spectrum shows a single
CO stretch at 1640 cm–1
. The 1
H NMR
spectrum is a single line even at low
temperatures. From this spectroscopic
information and the CVE, propose a
structure for [Fe4(Cp)4(CO)4]. TA
tetrahedron with 4 terminal Cp rings and four
capping COs.
S22.10 If Mo(CO)3L3 is desired, which of the
ligands P(CH3)3 or P(t-Bu)3 would be
preferred? Give reasons for your choice.
PMe3 would be preferred.
S22.11 Assess the relative substitutional
reactivities of indenyl and fluorenyl (86)
compounds? Fluorenyl compounds are more
reactive than indenyl.
S22.12 Show that the reaction is an example of
reductive elimination?
Pd
Ph PPh3
Ph3P Cl
Cl
Cl
Pd
Ph3P Cl
Cl PPh3
+ PhCl
The decrease in both coordination number and
oxidation number by 2.
S22.13 Explain why [Pt(PEt3)2(Et)(Cl)] readily
decomposes, whereas [Pt(PEt3)2 (Me)(Cl)]
does not? The ethyl group in
[Pt(PEt3)2(Et)(Cl)] is prone to β-hydride
elimination.
Exercises
22.1 Name the species, draw the structures of,
and give valence electron counts to the
metal atoms? Do any of the complexes
deviate from the 18-electron rule? If so,
how is this reflected in their structure or
chemical properties?
(a) Fe(CO)5? Pentacarbonyliron(0), 18e–
;
(b) Mn2(CO)10?
decacarbonyldimanganese(0), 18e–
;
(c) V(CO)6? hexcarbonylvanadium(0), 17e–
(d) [Fe(CO)4]2–
? tetracarbonylferrate(–2),
18e–
;
(e) La(η5
-Cp*)3?
tris(pentamethylcyclopentadienyl)lanthanum(I
II), 18e–
;
(f) Fe(η3
-allyl)(CO)3Cl?
allyltricarbonylchloroiron(II), 18e–
;
(g) Fe(CO)4(PEt3)?
tetracarbonyltriethylphosphineiron(0);
(h) Rh(CO)2(Me)(PPh3)?
dicarbonylmethyltriphenylphosphinerhodium(
I),16e-
(i) Pd(Cl)(Me)(PPh3)2?
chloromethylbis(triphenylphosphine)
palladium(II), 16e–
;
(j) Co(η5
-C5H5)(η4
-C4Ph4)?
cyclopentadienyltetraphenylcylcobutadinecob
alt(I), 18e–
;
(k) [Fe(η5
-C5H5)(CO2)]–
?
dicarbonylcyclopentadienylferrate(0), 18e–
;
(l) Cr(η6
-C6H6)(η6
-C7H8)?
benzenecycloheptatrienechromium(0), 18e–
;
(m) Ta(η5
-C5H5)2Cl3?
trichlorobiscyclopentadineyltanatalum(V),
18e–
;
(n) Ni(η5
-C5H5)NO?
cyclopentadineylnitrosylnickel(0),18e–
.
22.2 Sketch an η2
the interactions of 1,4-
butadiene with a metal atom and (b) do the
same for an η4
interaction.
22.3 What hapticities are possible for the
interaction of each of the following ligands
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-41-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES
42
with a single d-block metal atom such as
cobalt? (a) C2H4? η2
(b) Cyclopentadienyl? Can be η5
, η3
, or η1
.
(c) C6H6? η6
, η4
, and η2
.
(d) Cyclooctadiene? η2
and η4
.
(e) Cyclooctatetraene?η8
, η6
, η4
, η2
.
22.4 Draw plausible structures and give the
electron count of (a) Ni(η3
-C3H5)2 (b)
Co(η4
-C4H4)(η5
-C5H5) (c) Co(η3
-
C3H5)(CO)3. If the electron count deviates
from 18, is the deviation explicable in
terms of periodic terms.
(a) Ni(η3
-C3H5)2 16, very common for group
9 and group 10 elements.
(b) Co(η4
-C4H4)(η5
-C5H5)? 18.
(c) Co(η3
-C3H5)(CO)3? 18.
22.5 State the two common methods for the
preparation of simple metal carbonyls and
illustrate your answer with chemical
equations. Is the selection of method based
on thermodynamic or kinetic
considerations?
(1) Mo(s) + 6 CO(g) → Mo(CO)6(s) (high
temperature and pressure required)
(2) 2 CoCO3(s) + 2 H2(g) + 8 CO(g) →
Co2(CO)8(s) + 2 CO2 + 2 H2O
The reason that the second method is
preferred is kinetic.
22.6 Suggest a sequence of reactions for the
preparation of Fe(CO)3(dppe), given iron
metal, CO, dppe (Ph2PCH2CH2PPh2), and
other reagents of your choice.
Fe(s) + 5 CO(g) → Fe(CO)5(l) (high temperature
and pressure required)
Fe(CO)5(l) + diphos(s) → Fe(CO)3(diphos)(s) + 2
CO(g)
22.7 Suppose that you are given a series of
metal tricarbonyl compounds having the
respective symmetries C2v, D3h, and Cs.
Without consulting reference material,
which of these should display the greatest
number of CO stretching bands in the IR
spectrum? Check your answer and give the
number of expected bands for each by
consulting Table 22.7.
Cs symmetry complex, 3
22.8 Provide plausible reasons for the
differences in IR wavenumbers between each of the
following pairs:
(a) Mo(PF3)3(CO)3 2040, 1991 cm–1
versus
Mo(PMe3)3(CO)3 1945, 1851 cm–1
? CO
bands of the trimethylphosphine complex are
100 cm–1
or more lower in frequency. PMe3 is
primarily a σ-donor ligand. PF3 is primarily
a π-acid ligand.
(b) MnCp(CO)3 2023, 1939 cm–1
vs.
MnCp*(CO)3 2017, 1928 cm–1
? CO bands
of the Cp* complex are lower in frequency
than the corresponding bands of the Cp
complex. Cp* is a stronger donor ligand than
Cp.
22.9 The compound Ni3(C5H5)3(CO)2 has a
single CO stretching absorption at 1761
cm–1
. The IR data indicate that all C5H5
ligands are pentahapto and probably in
identical environments. (a) On the basis of
these data, propose a structure.
(b) Does the electron count for each metal
in your structure agree with the 18-
electron rule? If not, is nickel in a
region of the periodic table where
deviations from the 18-electron rule
are common? No. Deviations from the
rule are common for cyclopentadienyl
complexes to the right of the d block.
22.10 Decide which of the two complexes W(CO)6
or IrCl(CO)(PPh3)2 should undergo the
faster exchange with 13
CO. Justify your
answer. The Ir complex. It has an A
mechanism.
22.11 Which metal carbonyl in each of (a)
[Fe(CO)4]2–
or [Co(CO)4]–
(b) [Mn(CO)5]–
or [Re(CO)5]–
should be the most basic
toward a proton? What are the trends on
which your answer is based ?
(a) [Fe(CO)4]2–
The trend involved is the
greater affinity for a cation that a species with
a higher negative charge has.
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-42-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES 43
(b) The rhenium complex. The trend involved
is the greater M–H bond enthalpy for a period
6 metal ion relative to a period 4 metal ion in
the same group.
22.12 Using the 18-electron rule as a guide,
indicate the probable number of carbonyl
ligands in (a) W(η6
-C6H6)(CO)n, (b) Rh(η5
-
C5H5)(CO)n, and (c) Ru3(CO)n.
(a) 3 (b) 2 (c) 12
22.13 Propose two syntheses for MnMe(CO)5,
both starting with Mn2(CO)10, with one
using Na and one using Br2? You may use
other reagents of your choice. (i) Reduce
Mn2(CO)10 with Na to give Mn(CO)5
–
; react
with MeI to give MnMe(CO)5. (ii), Oxidize
with Br2. to give MnBr(CO)5; displace the
bromide with MeLi to give MnMe(CO)5.
22.14 Give the probable structure of the product
obtained when Mo(CO)6 is allowed to react
first with LiPh and then with the strong
carbocation reagent, CH3OSO2CF3.
Mo(CO)5(C(OCH3)Ph)
22.15 Na[W(η5
-C5H5)(CO)3] reacts with 3-
chloroprop-1-ene to give a solid, A, which
has the molecular formula
W(C3H5)(C5H5)(CO)3. Compound A loses
carbon monoxide on exposure to light and
forms compound B, which has the formula
W(C3H5)(C5H5)(CO)2. Treating compound
A with hydrogen chloride and then
potassium hexafluorophosphate, K+
PF6–
,
results in the formation of a salt, C.
Compound C has the molecular formula
[W(C3H6)(C5H5)(CO)3]PF6. Use this
information and the 18-electron rule to
identify the compounds A, B, and C.
Sketch a structure for each, paying
particular attention to the hapticity of the
hydrocarbon.
W
CO
CO
CO
A:
W
CO
CO
B:
W
CO
CO
CO
C:
η3
η2
η1
+
22.16 Treatment of TiCl4 at low temperature
with EtMgBr gives a compound that is
unstable above 270ºC. However, treatment
of TiCl4 at low temperature with MeLi or
LiCH2SiMe3 gives compounds that are
stable at room temperature. Rationalize
these observations.
The compounds formed are TiR4; ethyl has
the low energy β-hydride elimination
decomposition.
22.17 Suggest syntheses of (a) [Mo(η7
-
C7H7)(CO)3]BF4 from Mo(CO)6 and (b)
[IrCl2(COMe)(CO)(PPh3)2] from
[IrCl(CO)(PPh3)2]?
(a) Reflux Mo(CO)6 with cycloheptatriene to
give [Mo(η6
-C7H8)(CO)3]; treat with the trityl
tetrafluoroborate to give [Mo(η7
-
C7H7)(CO)3]BF4.
(b) React [IrCl(CO)(PPh3)2] with MeCl, then
expose to CO atmosphere to give
[IrCl2(COMe)(CO)(PPh3)2].
22.18 When Fe(CO)5 is refluxed with
cyclopentadiene compound A is formed
which has the empirical formula C8H6O3Fe
and a complicated 1
H NMR spectrum.
Compound A readily loses CO to give
compound B with two 1
H-NMR resonances,
one at negative chemical shift (relative
intensity one) and one at around 5ppm
(relative intensity 5). Subsequent heating of
B results in the loss of H2 and the
formation of compound C. Compound C
has a single 1
H-NMR resonance and the
empirical formula C7H5O2Fe. Compounds
A, B, and C all have 18 valence electrons:
identify them and explain the observed
spectroscopic data.
+ Fe(C O )5
Fe
O C C O
C O
-2C O -C O
F e
O C
C O
H
-H
A B
[π-C5H5Fe(CO)2]2
C
The compound B shows two 1
H NMR
resonances due to Fe-H proton and the
aromatic Cp ring. C shows a single 1
H NMR
resonance because of aromatic Cp ring.
22.19 When Mo(CO)6 is refluxed with
cyclopentadiene compound D is formed
which has the empirical formula
C8H5O3Mo and an absorption in the IR
spectrum at 1960 cm–1
. Compound D can
be treated with bromine to yield E or with
Na/Hg to give compound F. There are
absorptions in the IR spectra of E and F at
2090 and 1860 cm–1
, respectively.
Compounds D, E, and F all have 18 valence
electrons: identify them and explain the
observed spectroscopic data.
D = C5H5Mo(CO)3. E = C5H5Mo(CO)3Br.
F = C5H5Mo(CO)3Na.
22.20 Which compound would you expect to be
more stable,RhCp2 or RuCp2? Give a
plausible explanation for the difference in
terms of simple bonding concepts
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-43-320.jpg)
![ANSWERS TO SELF-TESTS AND EXERCISES
44
RuCp2 has 18 electrons.
22.21 Give the equation for a workable reaction
for the conversion of Fe(η5
-C5H5)2 to Fe(η5
-
C5H5) (η5
-C5H4COCH3) and (b) Fe(η5
-
C5H5) (η5
-C5H4CO2H)
(a) Fe(η5
– C5H5)2 + CH3COCl →
Fe(η5
– C5H5)(η5
– C5H4COCH3) + HCl
(b)
Fe +
C l O
C l
A lCl3
CH 2Cl2
Fe
O
C l
H 2O , t-B uO K
D M E
Fe
C O 2H
22.22 Sketch the a1’ symmetry-adapted orbitals
for the two eclipsed C5H5 ligands stacked
together with D5h symmetry. Identify the s,
p, and d orbitals of a metal atom lying
between the rings that may have nonzero
overlap, and state how many a1’ molecular
orbitals may be formed.
The symmetry-adapted orbitals of the two
eclipsed C5H5 rings in a metallocene are
shown in Resource Section 5, the dz2 orbital
on the metal has a1′ symmetry, as does s.
Three a1′ MOs will be formed.
22.23 The compound Ni(η5
-C5H5)2 readily adds
one molecule of HF to yield [Ni(η5
-
C5H5)(η4
-C5H6)]+
whereas Fe(η5
-C5H5)2
reacts with strong acid to yield [Fe(η5
-
C5H5)2H]+
. In the latter compound the H
atom is attached to the Fe atom. Provide a
reasonable explanation for this difference.
Protonation of FeCp2 at iron does not change
its number of valence electrons.
22.24 Write a plausible mechanism, giving your
reasoning, for the reactions: (a)
[Mn(CO)5(CF2)]+
+ H2O → [Mn(CO)6]+
+
2HF?
(i) The F atoms render the C atom subject to
nucleophilic attack
(ii)two equivalents of HF are eliminated
(b) Rh(C2H5) (CO) (PR3)2 →
RhH(CO)(PR3)2 + C2H4? A β-hydrogen
elimination reaction.
22.25 Given mechanism of CO insertion, what
rate constant can be extracted from rate
data? Rate = ka[RMn(CO)5]
22.26 (a) What cluster valence electron (CVE)
count is characteristic of octahedral and
trigonal prismatic complexes?
octahedral M6, 86; trigonal prismatic M6, 90.
(b) Can these CVE values be derived from
the 18-electron rule? No.
(c) Determine the probable geometry of
[Fe6(C)(CO)16]2–
and [Co6(C)(CO)15]2-
? The iron complex probably contains an
octahedral Fe6 array. The cobalt complex
probably contains a trigonal-prismatic Co6
array.
22.27 Based on isolobal analogies, choose the
groups that might replace the group in
boldface in (a) Co3(CO)9CH→ OCH3,
N(CH3)2, or SiCH3? SiCH3.
(b) (OC)5MnMn(CO)5 → I, CH2, or
CCH3? I.
22.28 Ligand substitution reactions on metal
clusters are often found to occur by associative
mechanisms, and it is postulated that these occur by
initial breaking of an M-M bond, thereby providing
an open coordination site for the incoming ligand. If
the proposed mechanism is applicable, which would
you expect to undergo the fastest exchange with
added13
CO, Co4(CO)12 or Ir4(CO)12? Suggest an
explanation. Co. This is because metal–metal bond
strengths increase down a group in the d block.
CHAPTER 23
Self-tests
S23.1 Derive the ground state of the Tm3+
ion
3
H6.
S23.2 The product of the reaction above is in fact
a hydride bridged dimer (9). Suggest a
strategy to ensure that the hydride is
monomeric. A simple approach would be to
use the Cp rings substituted by bulky groups
and examine rate of formation.
S23.3 Use the Frost diagrams and data in
Resource section 2 to determine the most
stable uranium ion in acid aqueous solution
in the presence of air and give its
formula. UO2
2+
if sufficient oxygen is
present.
Exercises
23.1 (a) Give a balanced equation for the
reaction of any of the lanthanoids with
aqueous acid. (b) Justify your answer with
redox potentials and with a generalization
on the most stable positive oxidation states
for the lanthanoids. (c) Name the two
lanthanoids that have the greatest tendency
to deviate from the usual positive oxidation
Shriver & Atkins: Inorganic Chemistry 5e](https://image.slidesharecdn.com/answerstoself-testsandexercises-230805182846-120e13ad/85/Answers-To-Self-Tests-And-Exercises-44-320.jpg)