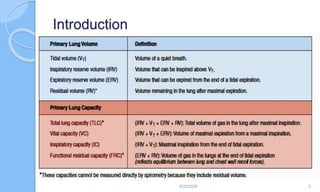
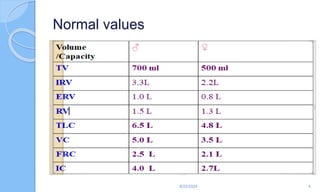
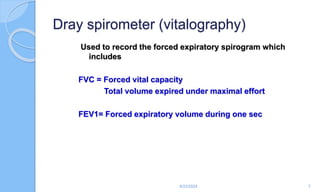
The document outlines the definition, causes, symptoms, and management of asthma, including specific preoperative, intraoperative, and postoperative considerations. It details pulmonary function tests, treatment protocols for acute asthma attacks, and best practices for anesthesia management in asthmatic patients. The material emphasizes the importance of patient evaluation and individualized treatment plans to optimize respiratory function during surgical procedures.