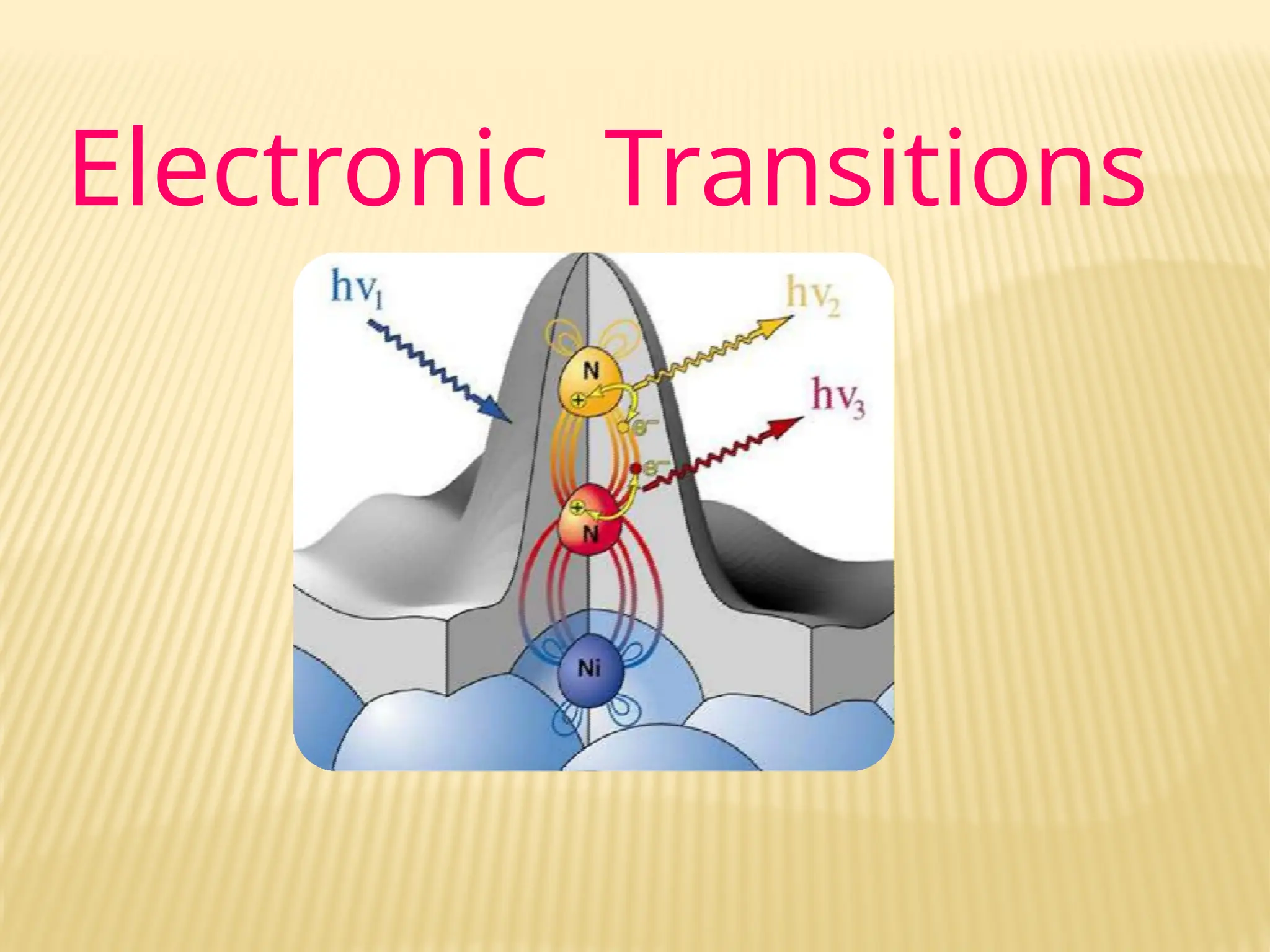
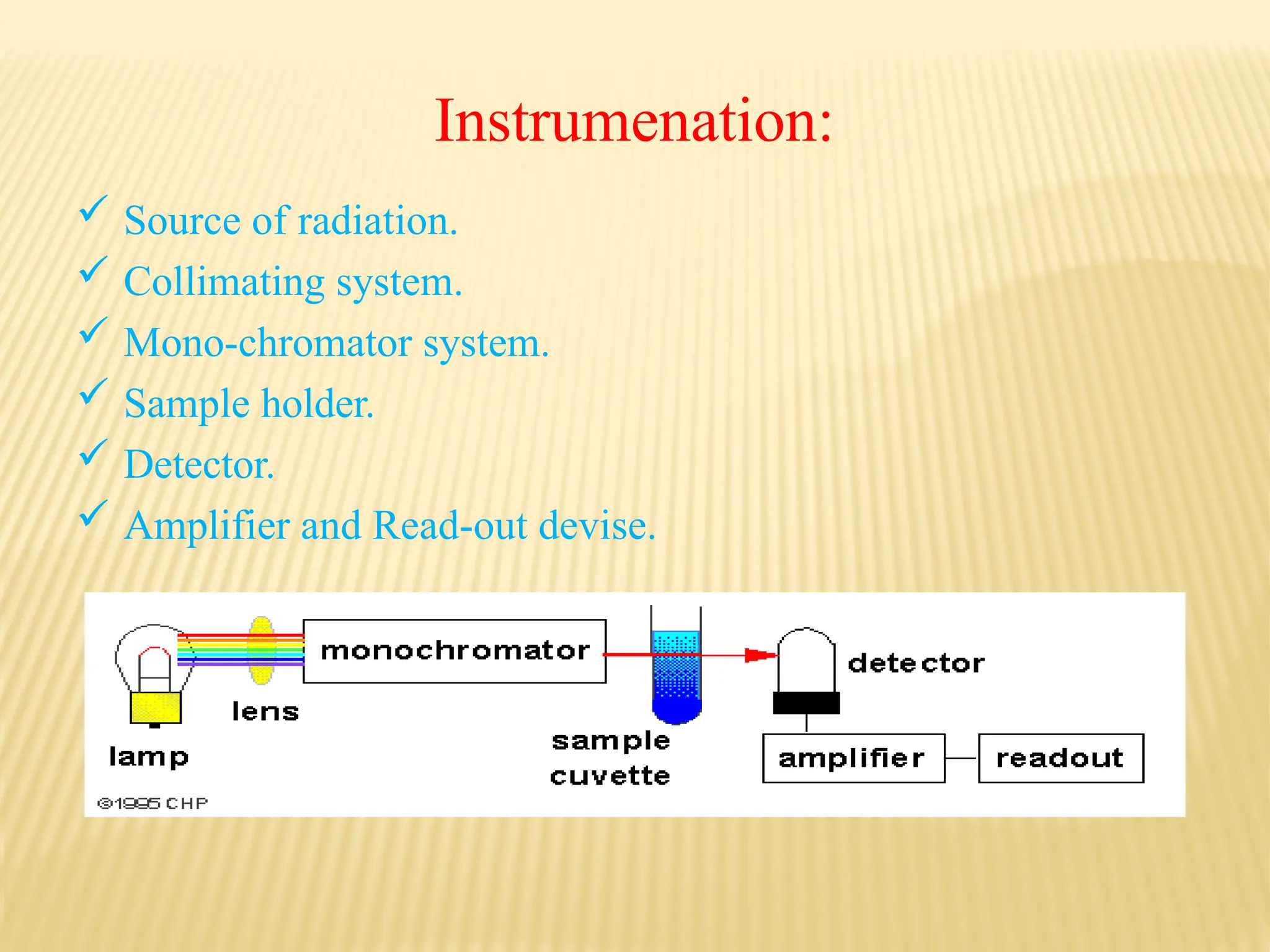
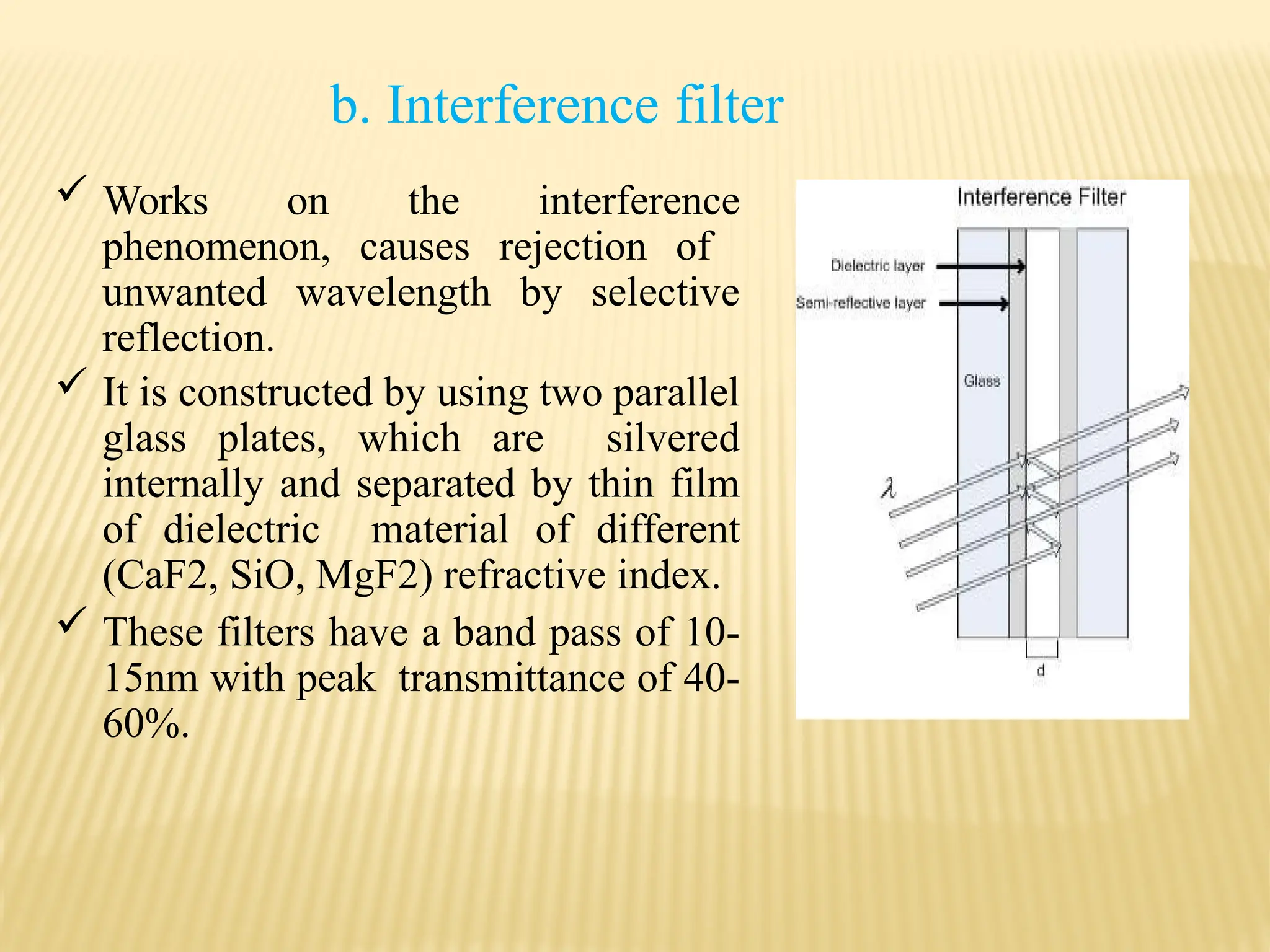
The document provides an overview of UV-visible spectroscopy, detailing its theory, principles, and instrumentation used to analyze the interaction of light with matter. It explains the significance of UV spectroscopy in detecting functional groups, impurities, and conducting quantitative and qualitative analysis. Key components such as the source of radiation, monochromators, sample holders, and detectors are also discussed, along with various applications in the field of pharmacy and chemistry.