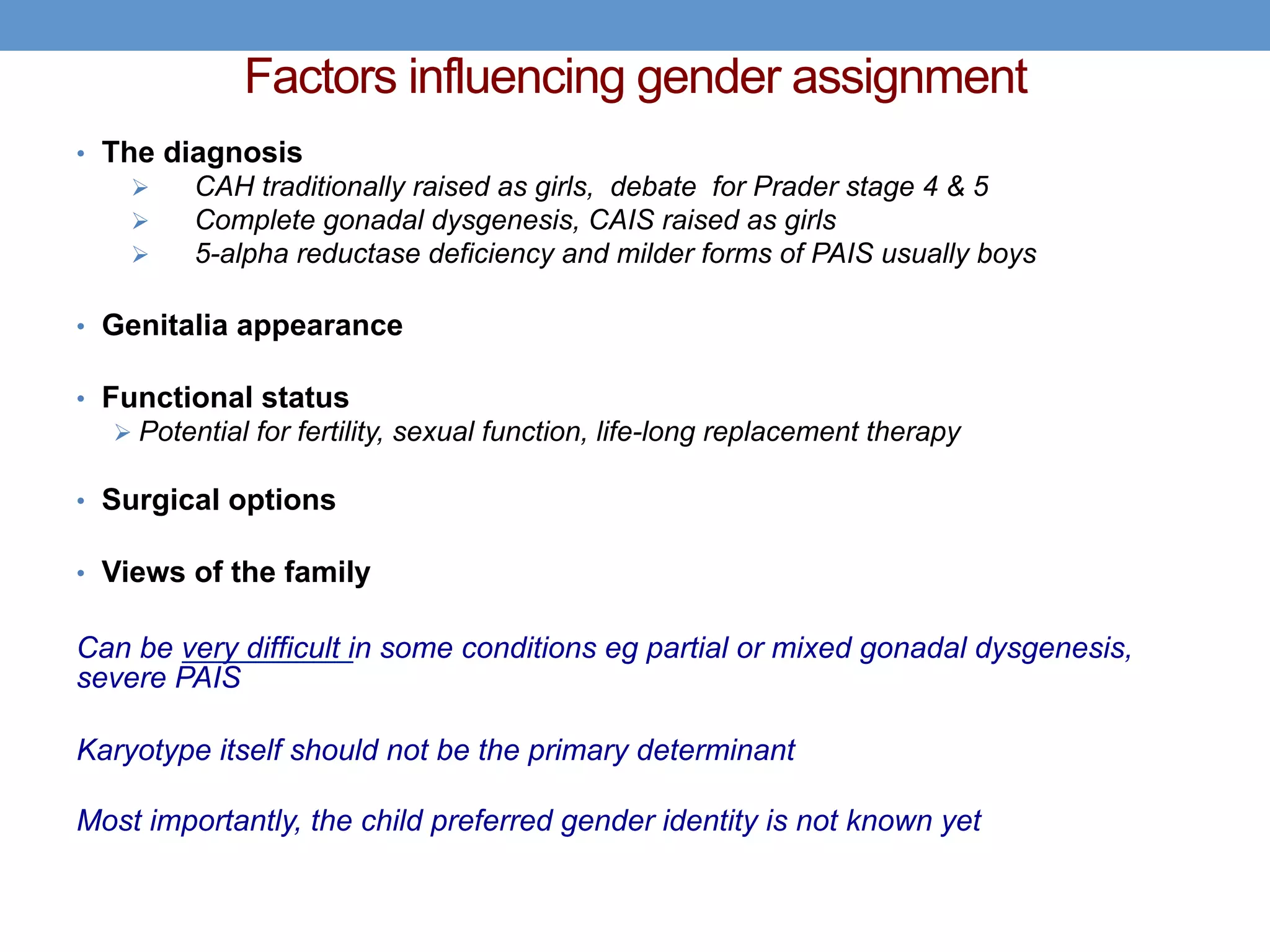
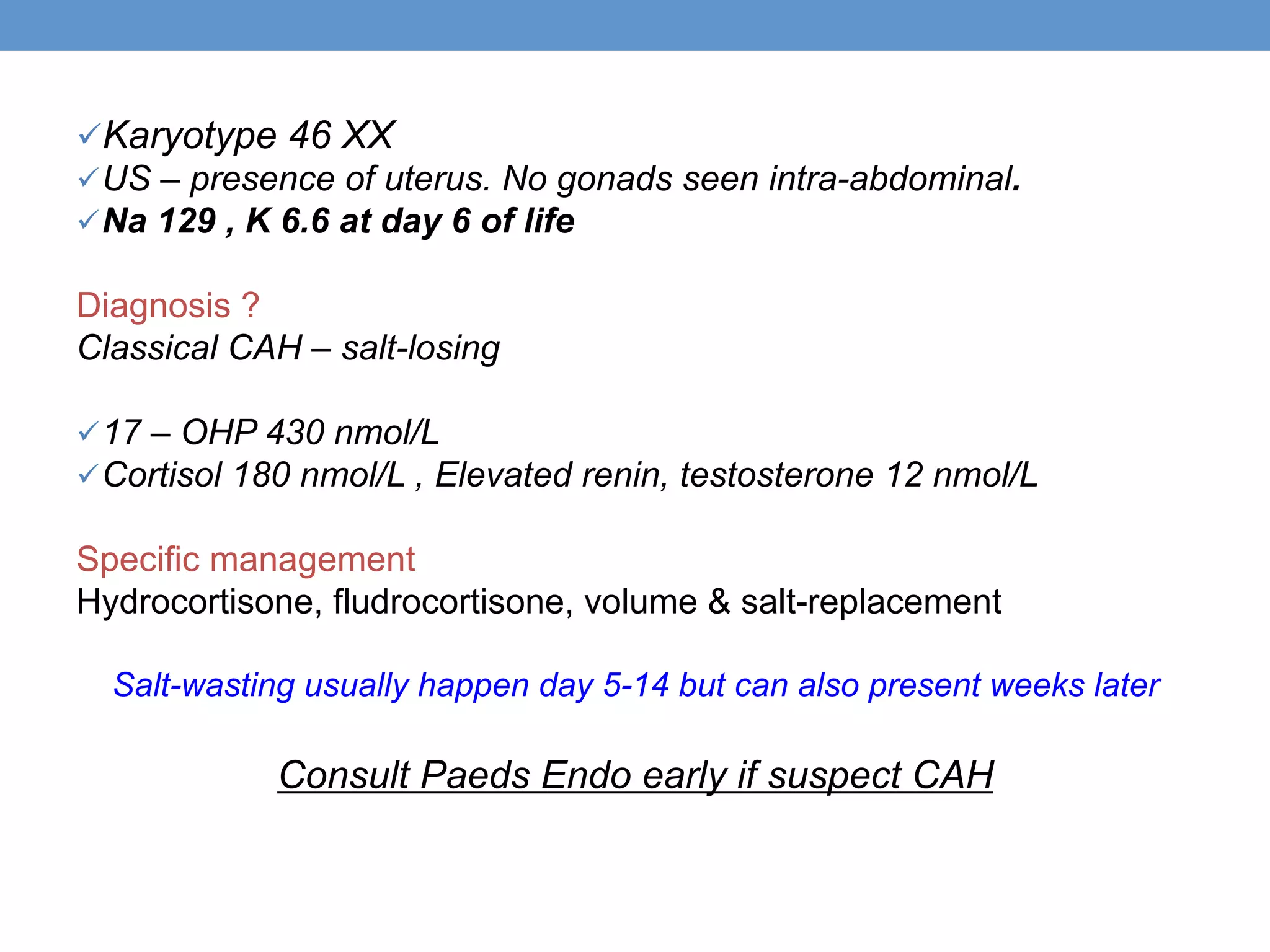
1. Ambiguous genitalia can be complicated to evaluate and manage, requiring a multidisciplinary approach.
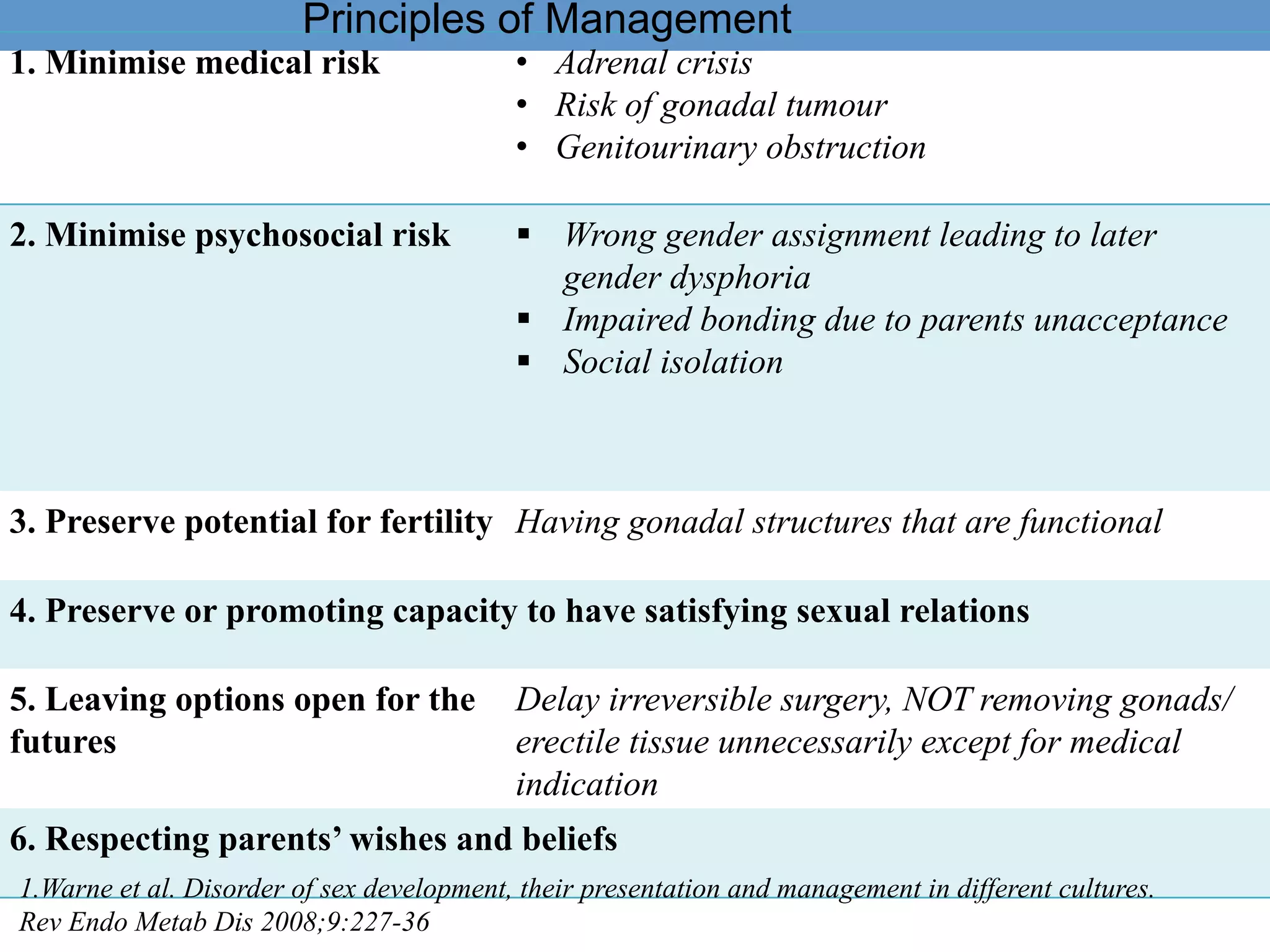
2. The initial assessment involves determining the need for investigation, ruling out life-threatening conditions like congenital adrenal hyperplasia, and assessing the history, examination, and basic lab tests to determine the underlying cause.
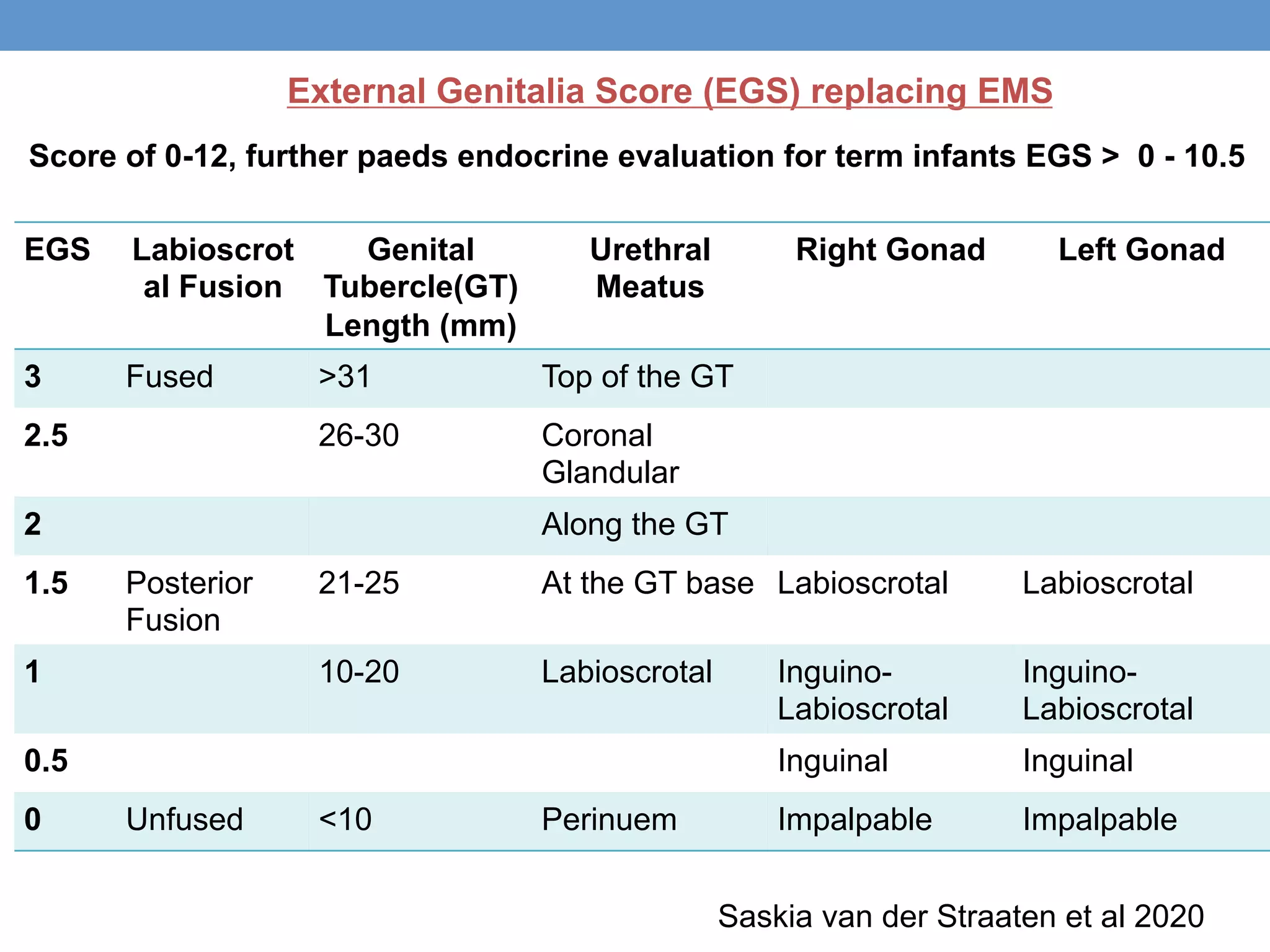
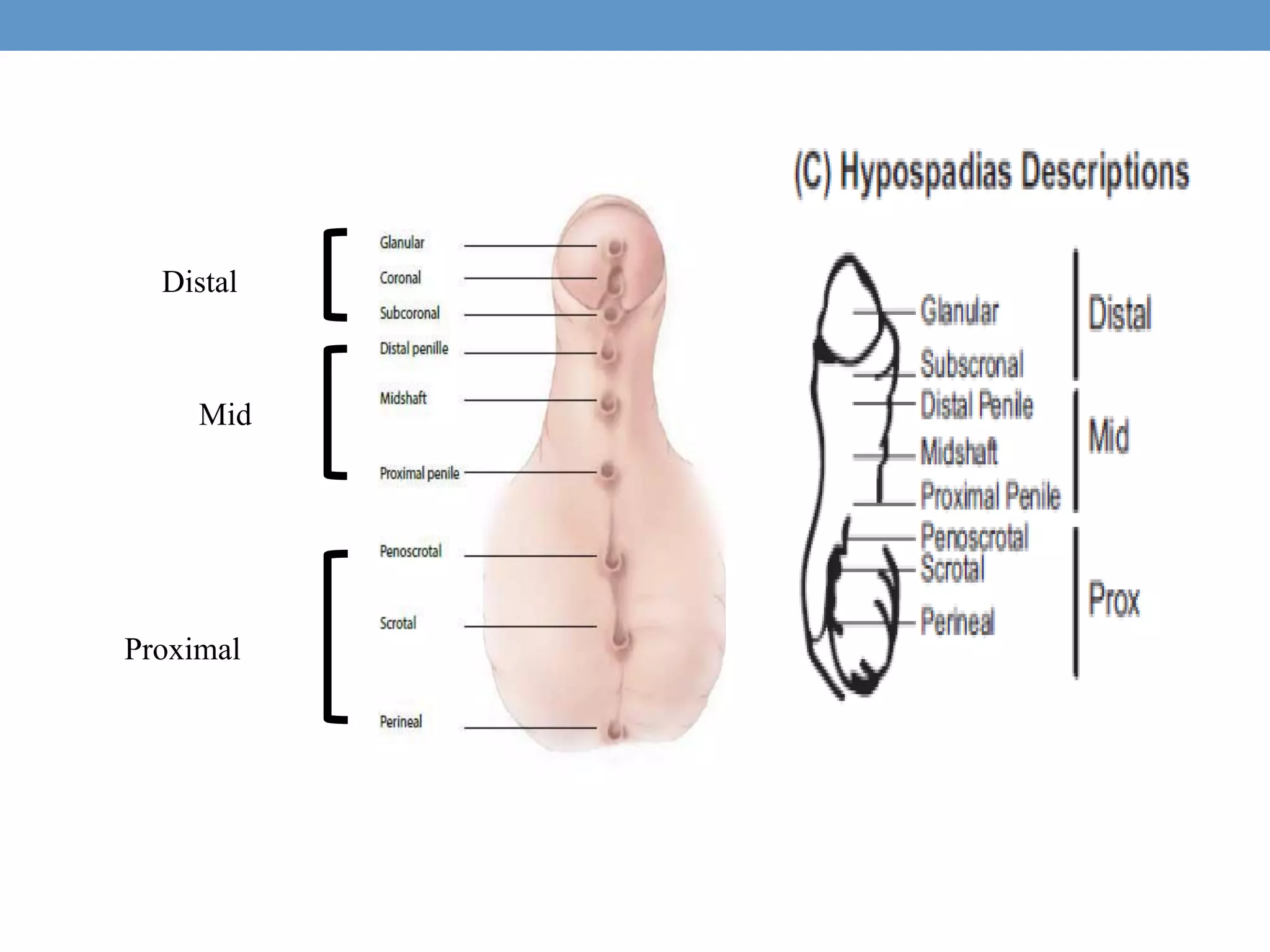
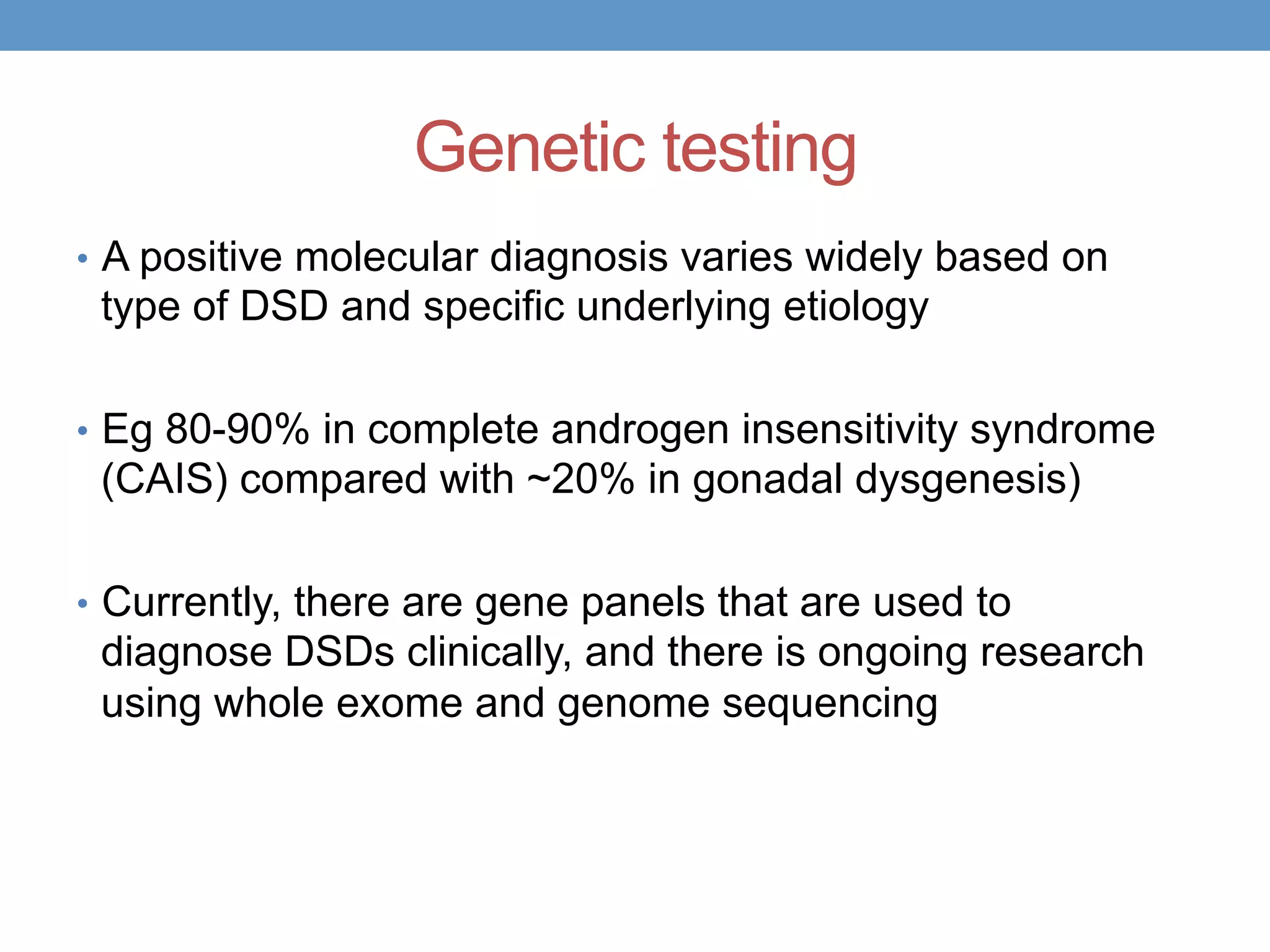
3. Further investigation may include karyotyping, genetic testing, imaging, and hormone level analysis to classify the condition, locate gonads, and assess gonadal and adrenal function.