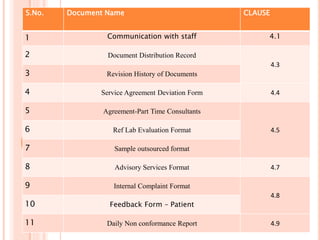
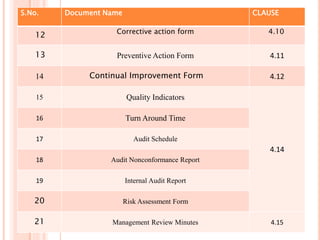
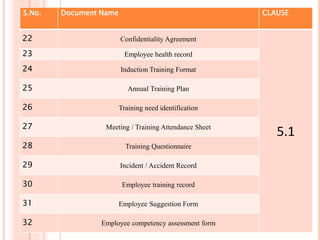
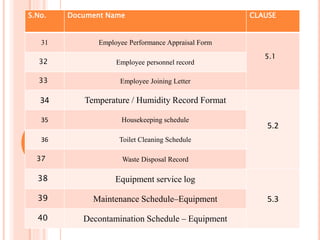
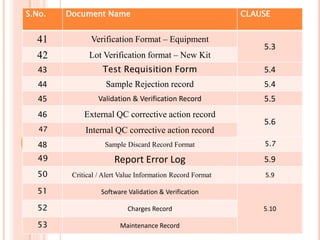
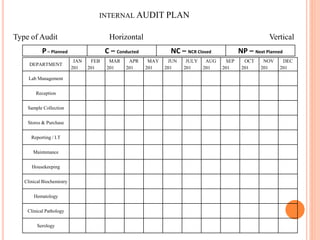
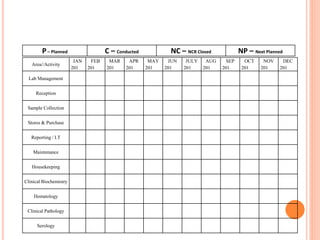
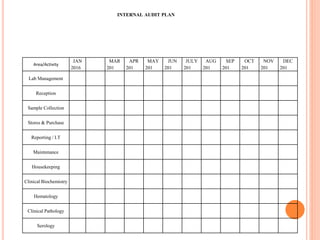
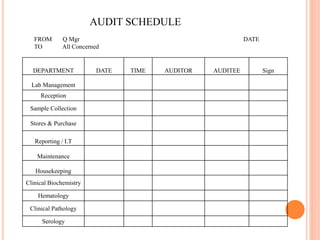
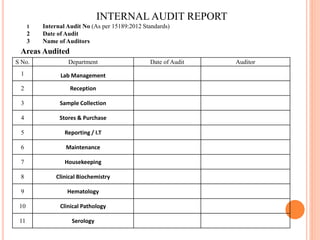
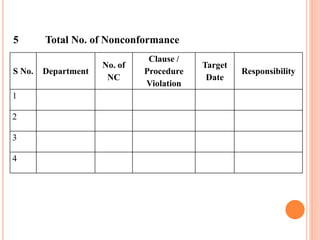
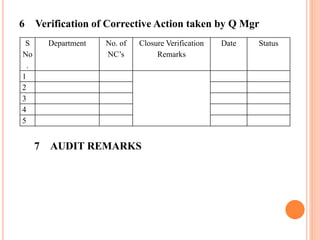
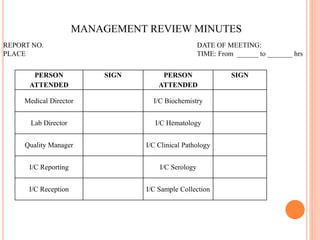
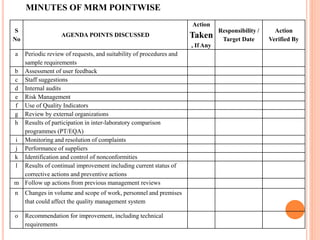
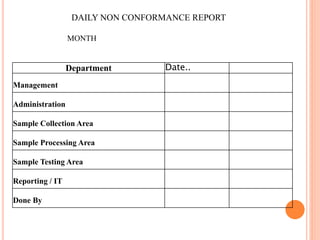
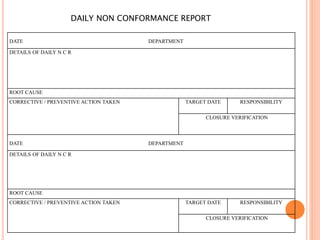
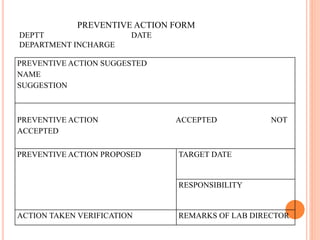
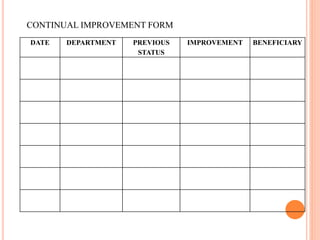
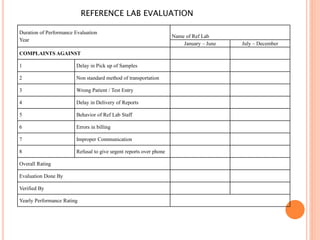
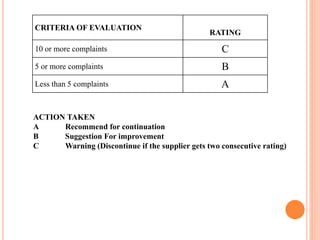
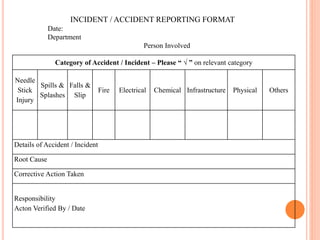
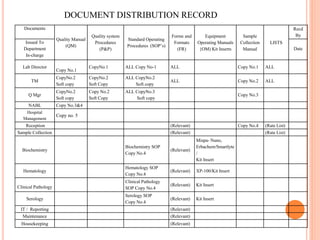
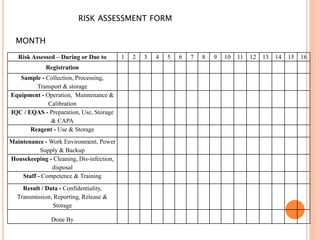
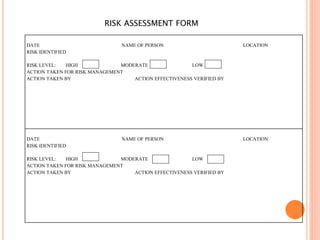
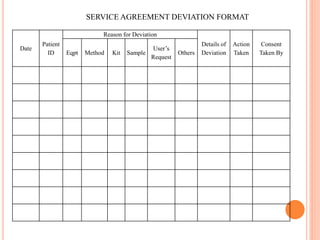
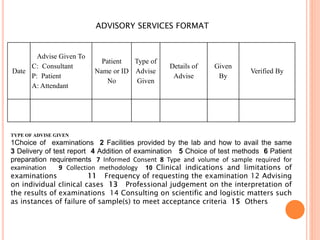
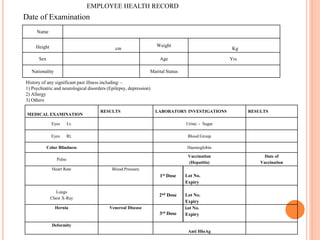
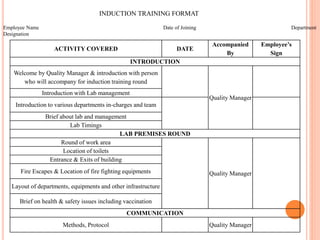
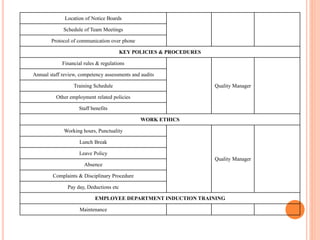
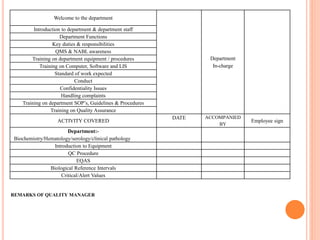
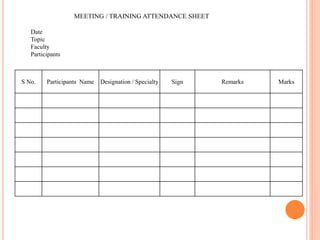
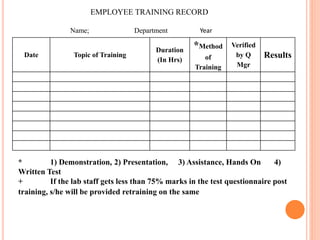
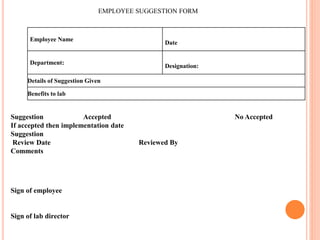
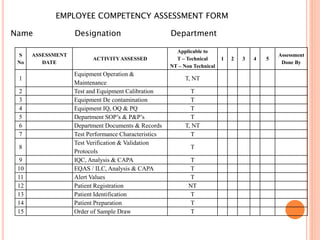
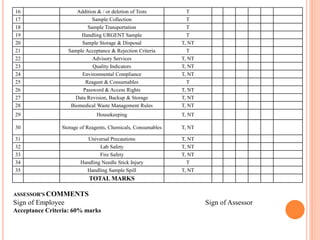
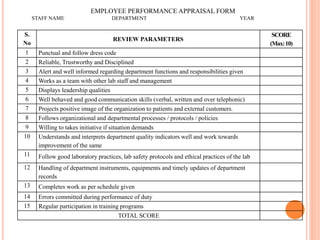
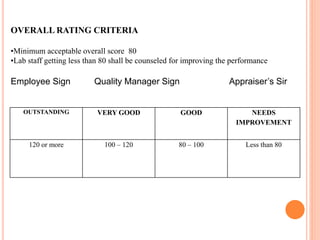
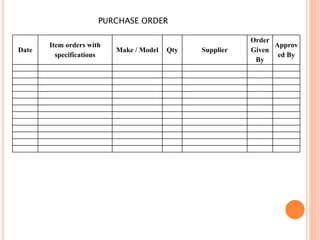
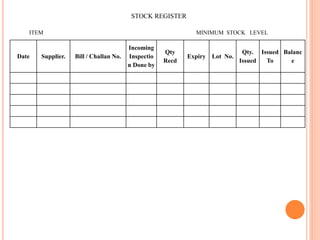
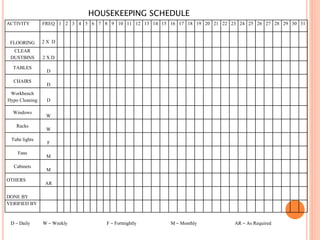
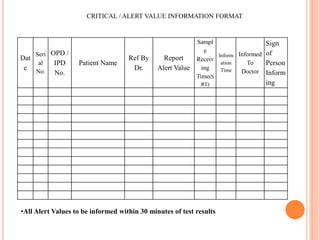
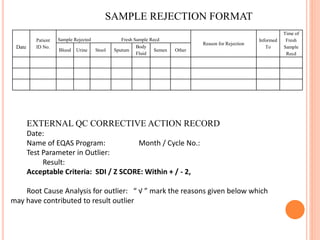
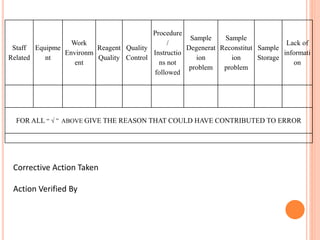
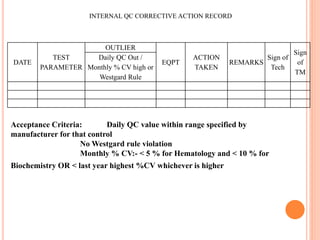
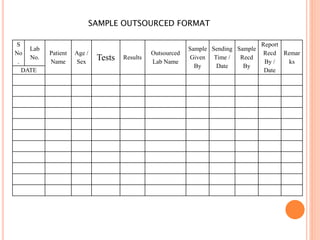
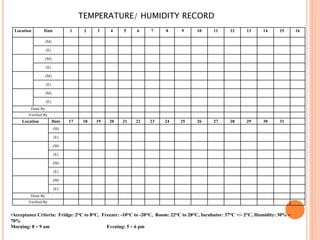
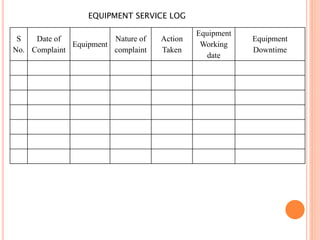
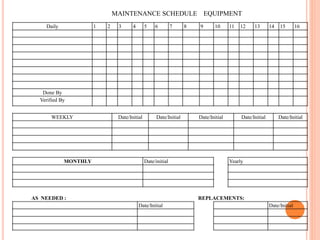
Dr. Neeraj has provided a list of records and formats necessary to meet ISO standards for laboratories. The list includes over 50 documents across various areas like management, employees, equipment, quality control, audits and more. Standard operating procedures and formats are given for communication, document control, service agreements, evaluations, audits, training and more. The laboratory may use these suggested formats or modify them as needed and add more over time to maintain compliance with ISO standards.