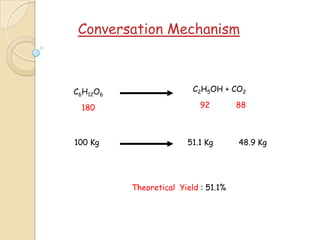
Alcohol fermentation is a biological process where sugars such as glucose and fructose are converted by yeasts into cellular energy and the waste products ethanol and carbon dioxide. Yeasts perform this conversion in the absence of oxygen, making it an anaerobic process. Common organisms used include various species of yeast and bacteria. Sugars, starches, and cellulosic materials can all serve as substrates. The process takes place under controlled temperature, pH, and time conditions to yield ethanol, which has various uses including as a fuel, preservative, solvent, and in alcoholic beverages.