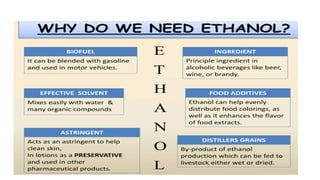
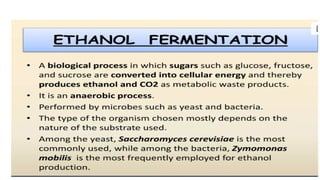
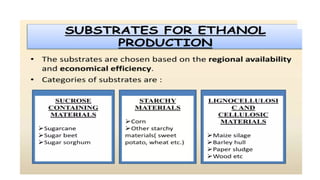
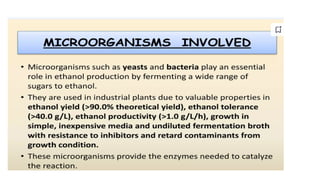
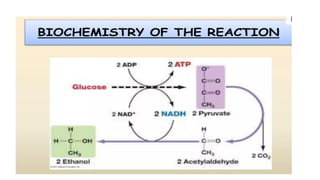
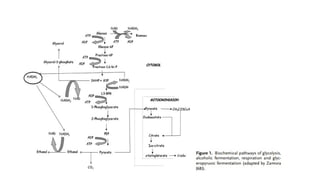
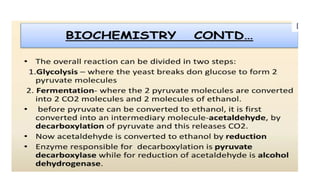
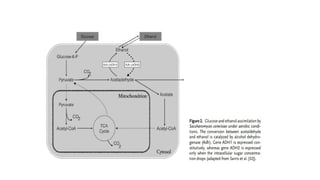
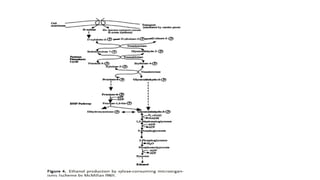
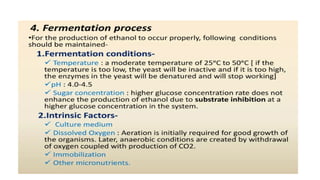
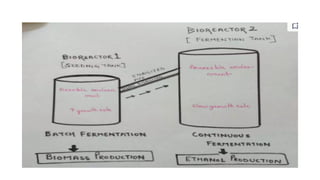
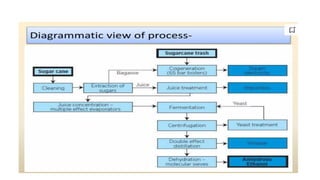
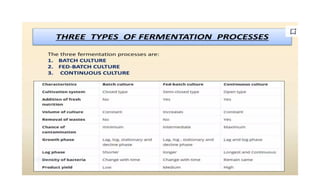
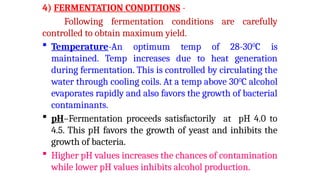
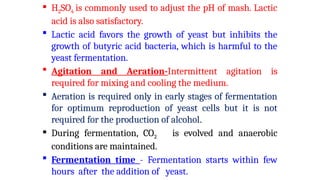
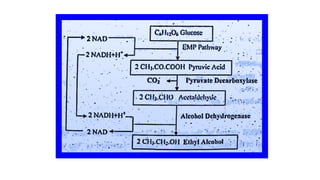
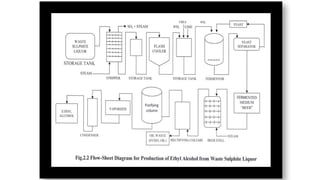
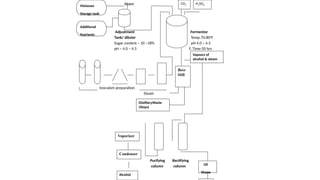
The document details the industrial production of ethyl alcohol primarily from molasses through a fermentation process involving yeast, including specific steps like inoculum preparation, fermentation medium setup, and environmental conditions control. It also outlines the recovery of alcohol and by-products, such as CO2, yeast for animal feed, fusel oils for the perfume industry, and slops used as fertilizers. Additionally, it discusses the fermentation process of waste sulphite liquor, emphasizing the necessity of removing toxic substances prior to utilizing it for alcohol production.