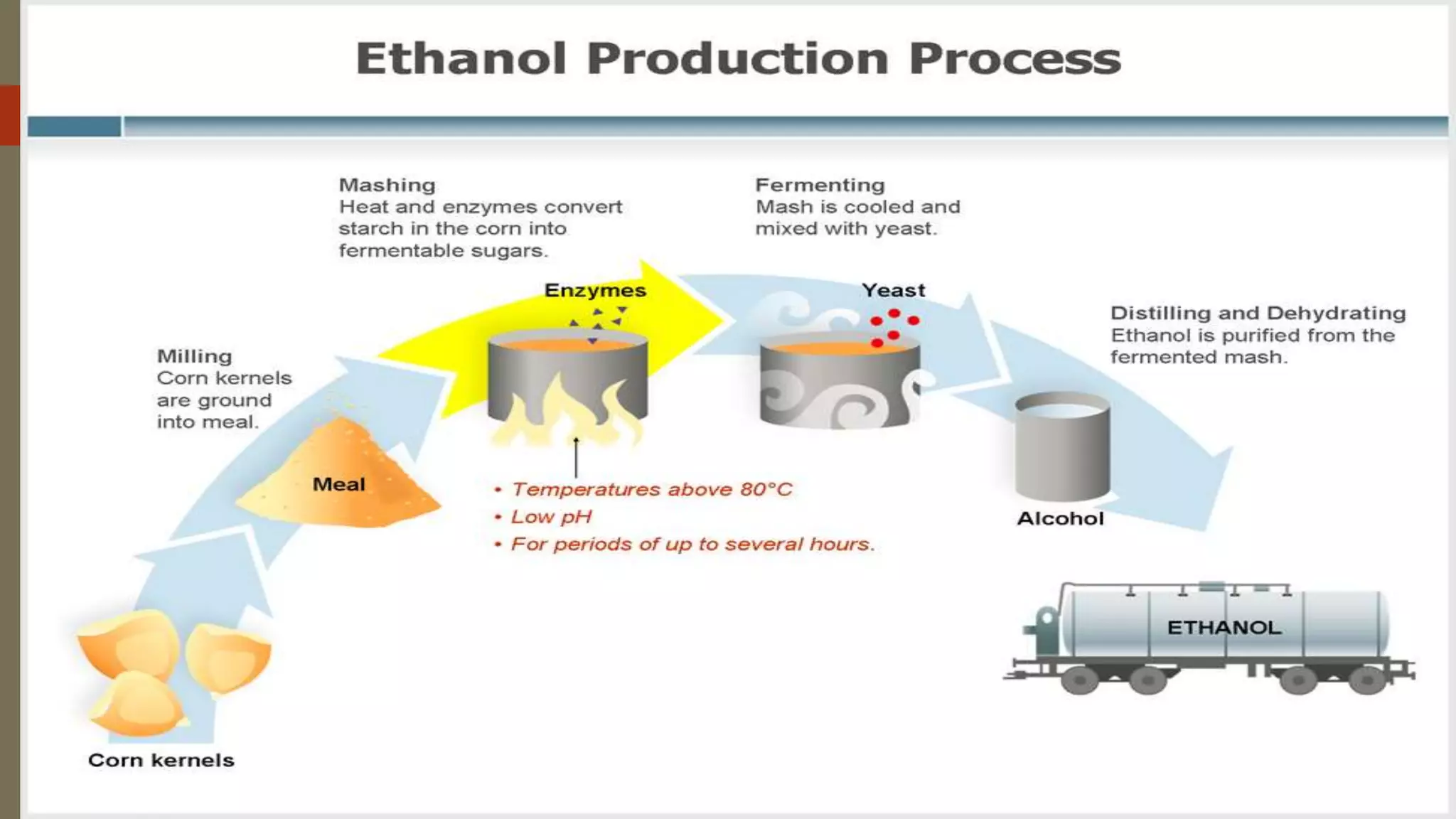
Ethanol, also known as ethyl alcohol fuel, is primarily produced through the fermentation of agricultural feedstocks like corn and sugarcane, making it a renewable energy source. The production process involves key steps such as milling, mashing, fermentation, distillation, and dehydration. Ethanol offers benefits such as cleaner combustion compared to gasoline but has disadvantages including lower energy density and higher production costs.







![ Advantages of Ethanol:
Ethanol burns cleaner than gasoline, reducing Green House Gases (GHG) emissions.
Ethanol does not contain significant amounts of toxic materials such as lead (Pb) and benzene (C6H6).
It is a Renewable source of energy extracted from plants.
All petrol engines can utilize the mixture of ethanol and needs no alterations. This decrease the
emission of hydrocarbons that deplete the ozone layer.
Disadvantages of Ethanol:
Lower heat of combustion.
Requires lot of land to be produced.
More expensive than MTBE ( Methyl Tert-Butyl Ether [CH3)3COCH3 ] ) or gasoline.
Decreases the mileage of vehicles.
ADVANTAGES &
DISADVANTAGES](https://image.slidesharecdn.com/ethanolproduction-191029135932/75/Ethanol-production-9-2048.jpg)

