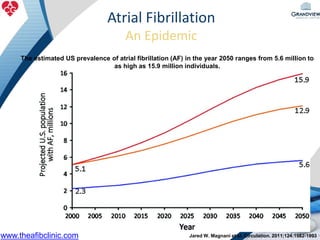
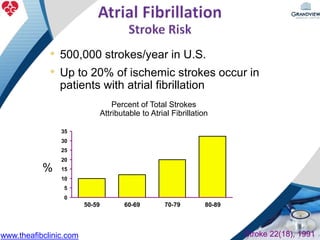
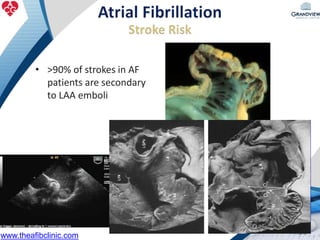
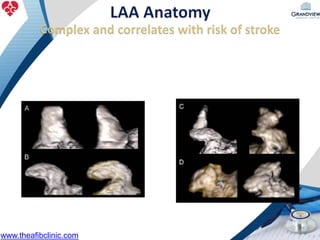
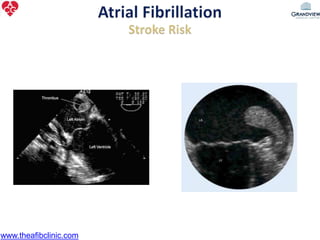
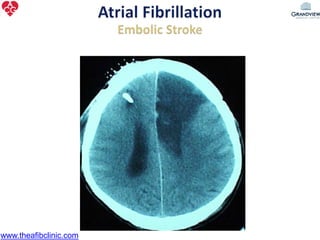
1) The document discusses atrial fibrillation (AF), its increasing prevalence, and its association with increased risk of stroke.
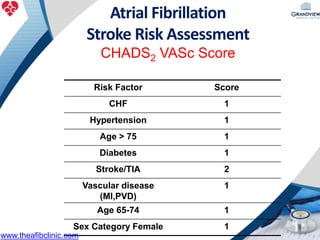
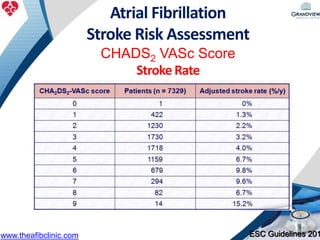
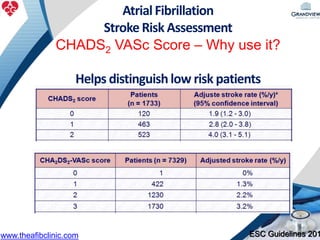
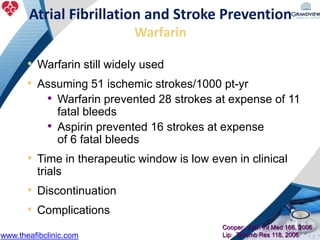
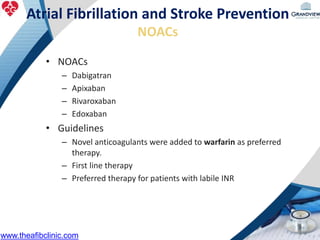
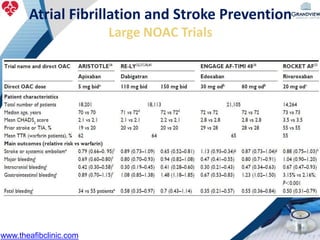
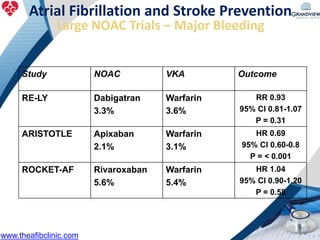
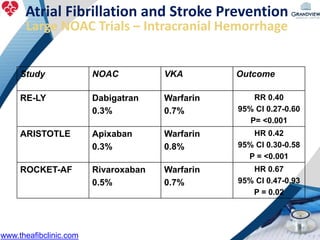
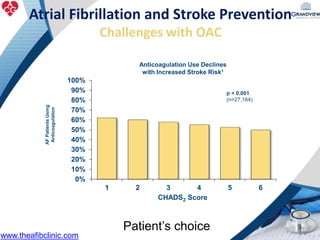
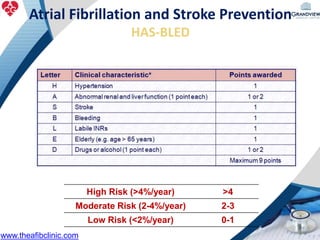
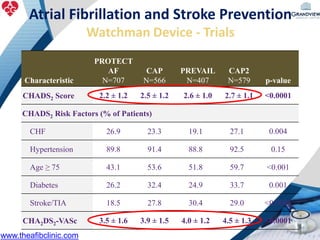
2) It reviews stroke risk assessment tools like CHADS2 and CHA2DS2-VASc scores and guidelines for stroke prevention in AF patients using anticoagulation or the newer oral anticoagulants (NOACs).
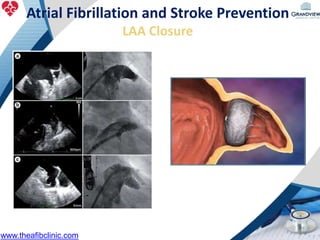
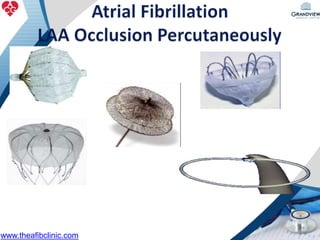
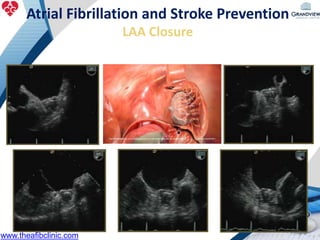
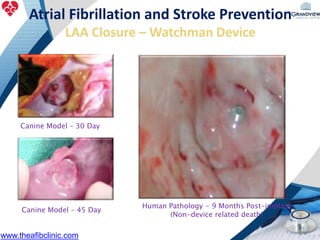
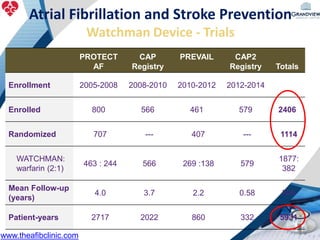
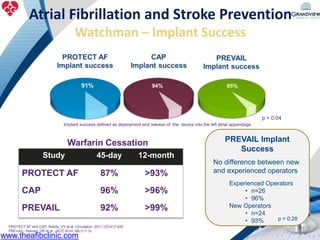
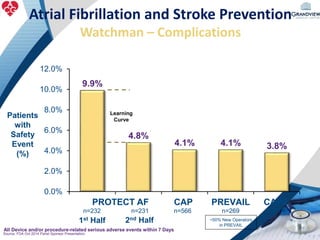
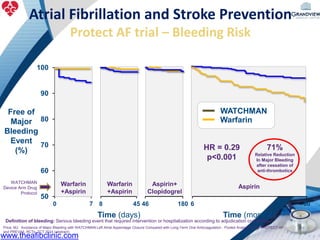
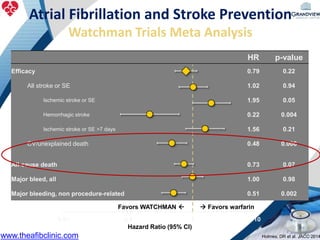
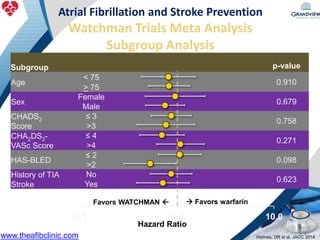
3) It also discusses left atrial appendage closure with the Watchman device as an alternative for stroke prevention in patients who cannot tolerate long-term anticoagulation. The Watchman trials demonstrated the device's safety and efficacy in reducing stroke risk comparable to warfarin.