Embed presentation
Downloaded 38 times
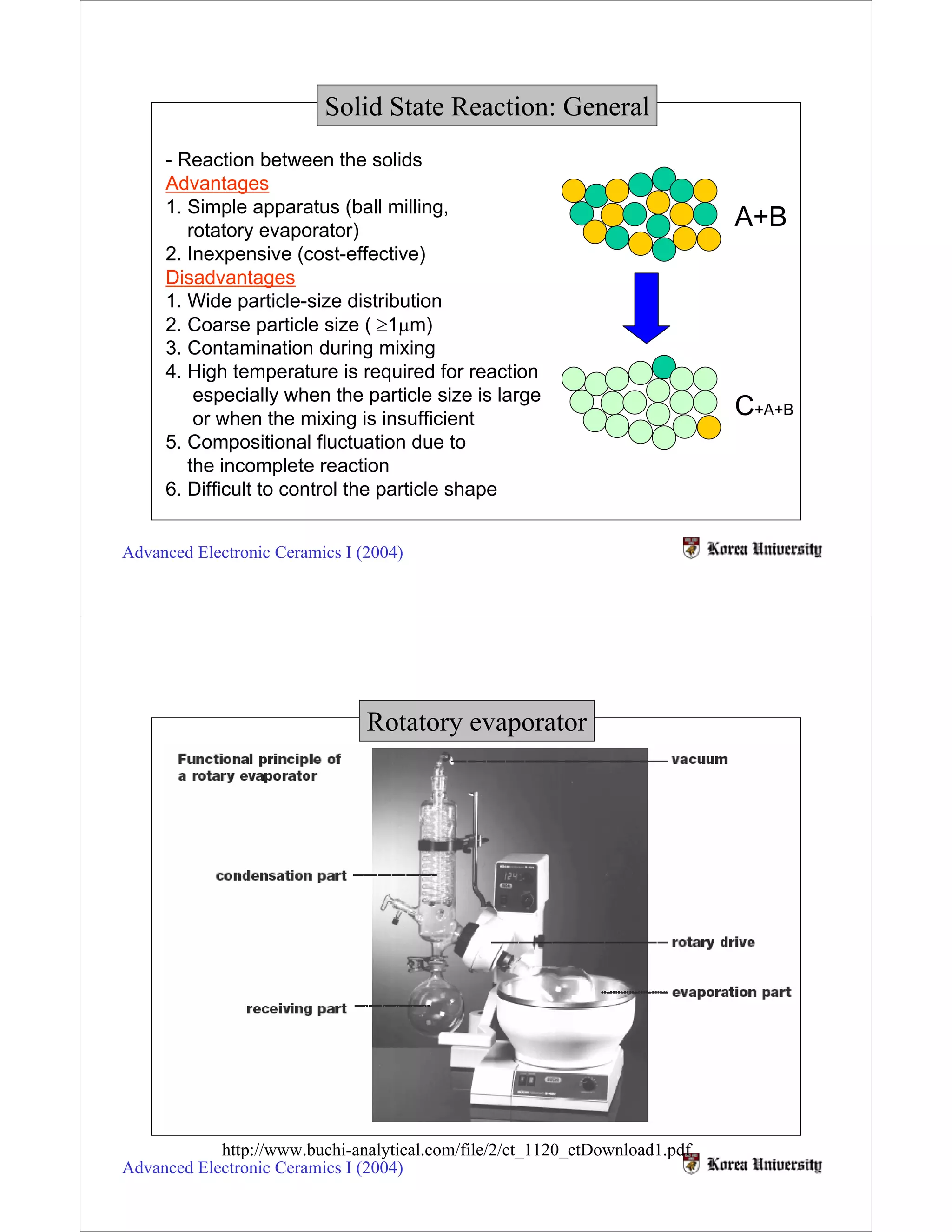
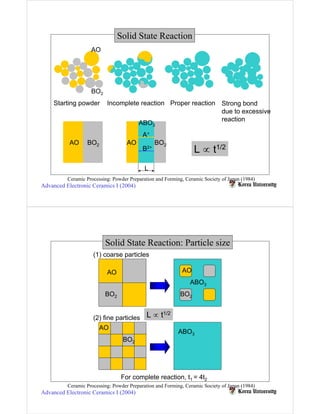
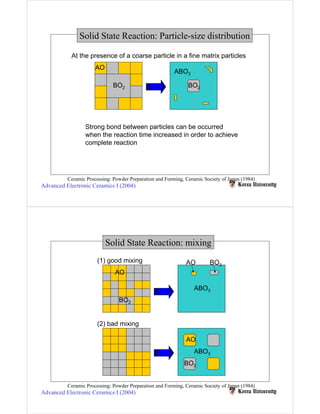

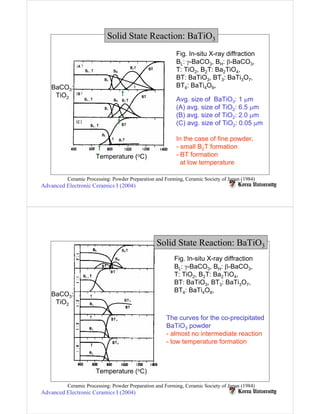

Solid state reactions involve reacting solids to form new solids and compounds. They have advantages like simple apparatus and low cost but also disadvantages like wide particle size distributions, need for high temperatures, and risk of contamination. Key factors that influence solid state reactions include particle size and distribution, mixing homogeneity, compaction between particles, and reaction temperature. Finer particle sizes and narrower distributions can promote more complete reactions at lower temperatures by increasing contact points between reactants.





