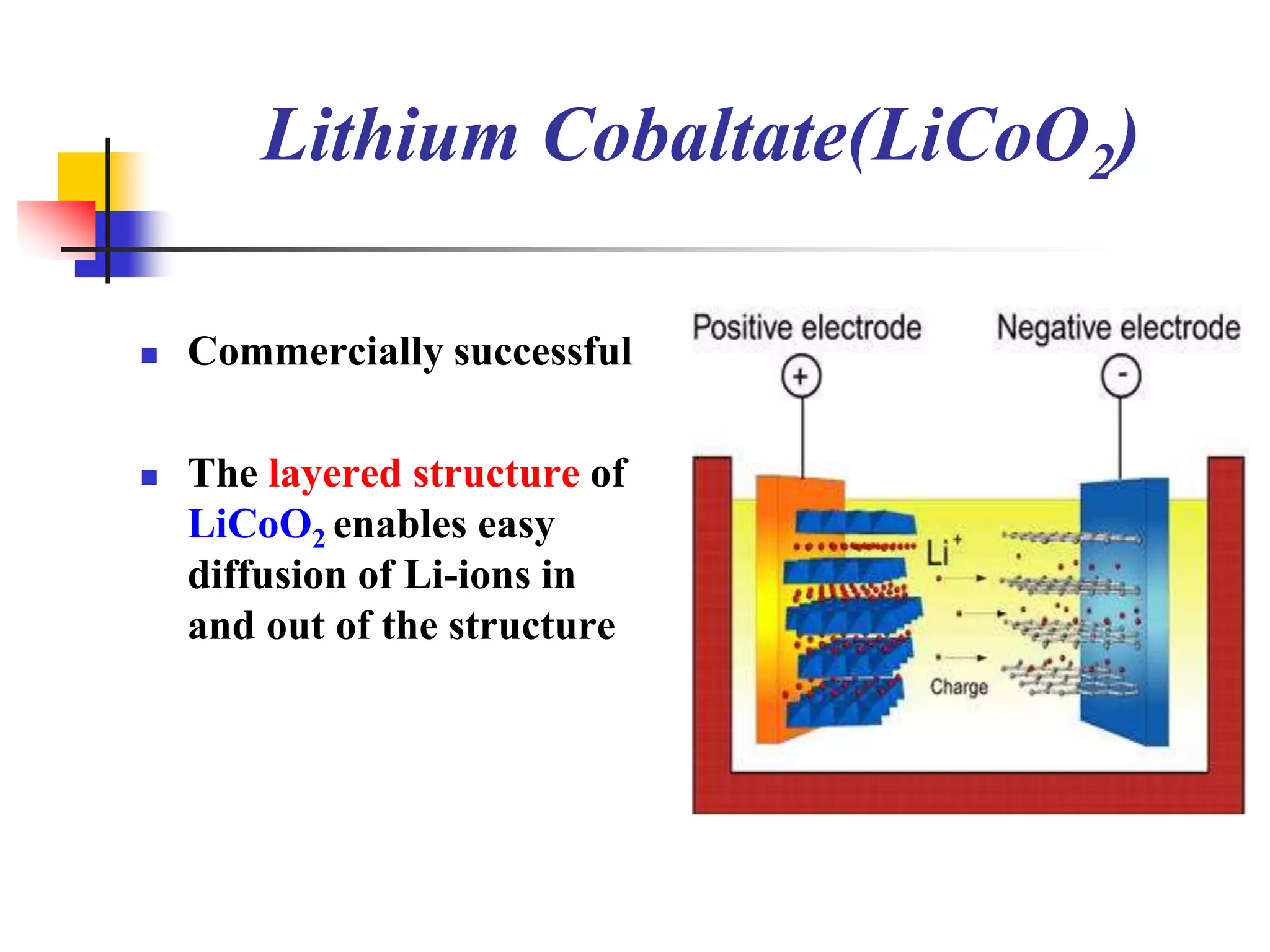
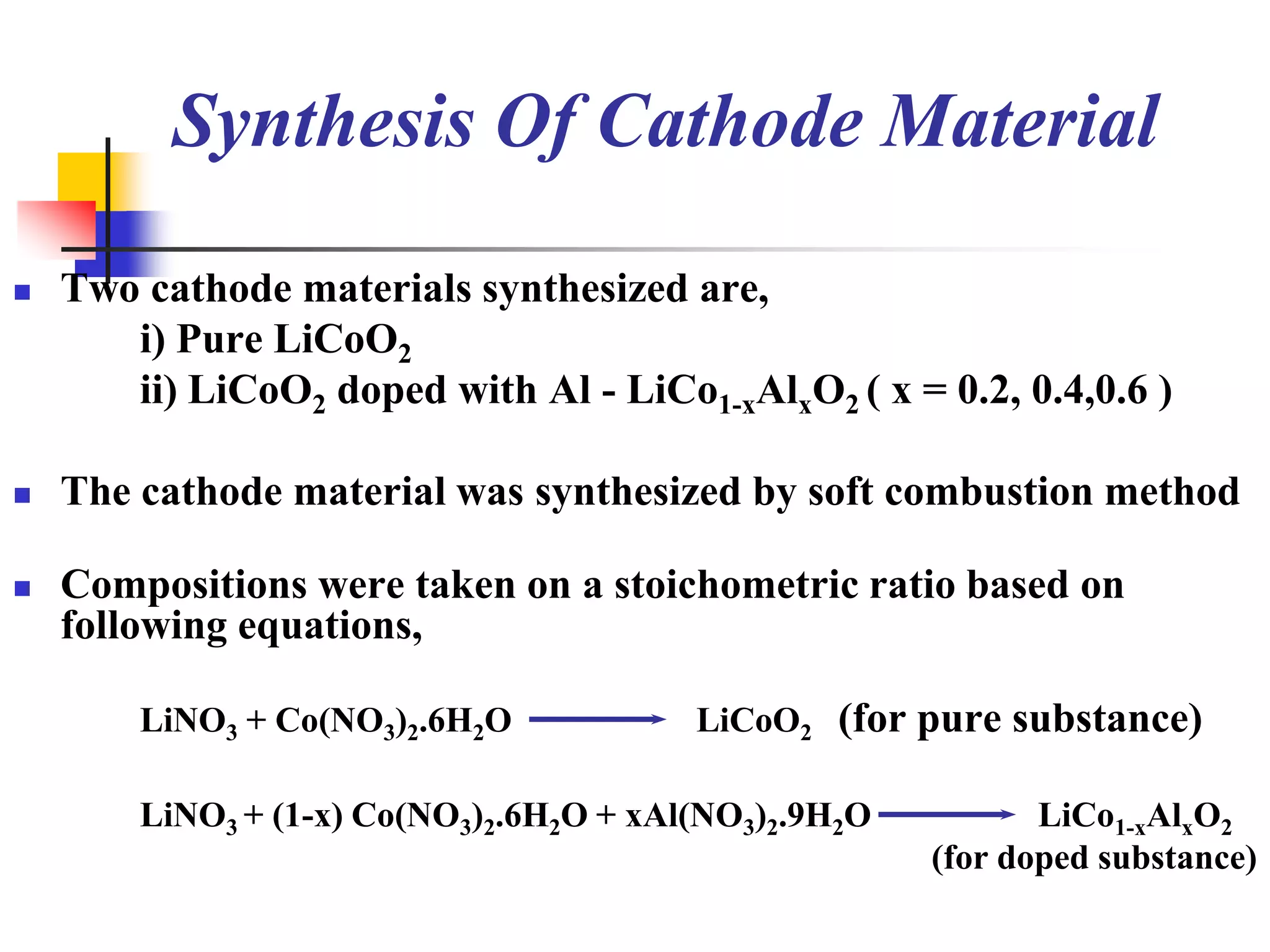
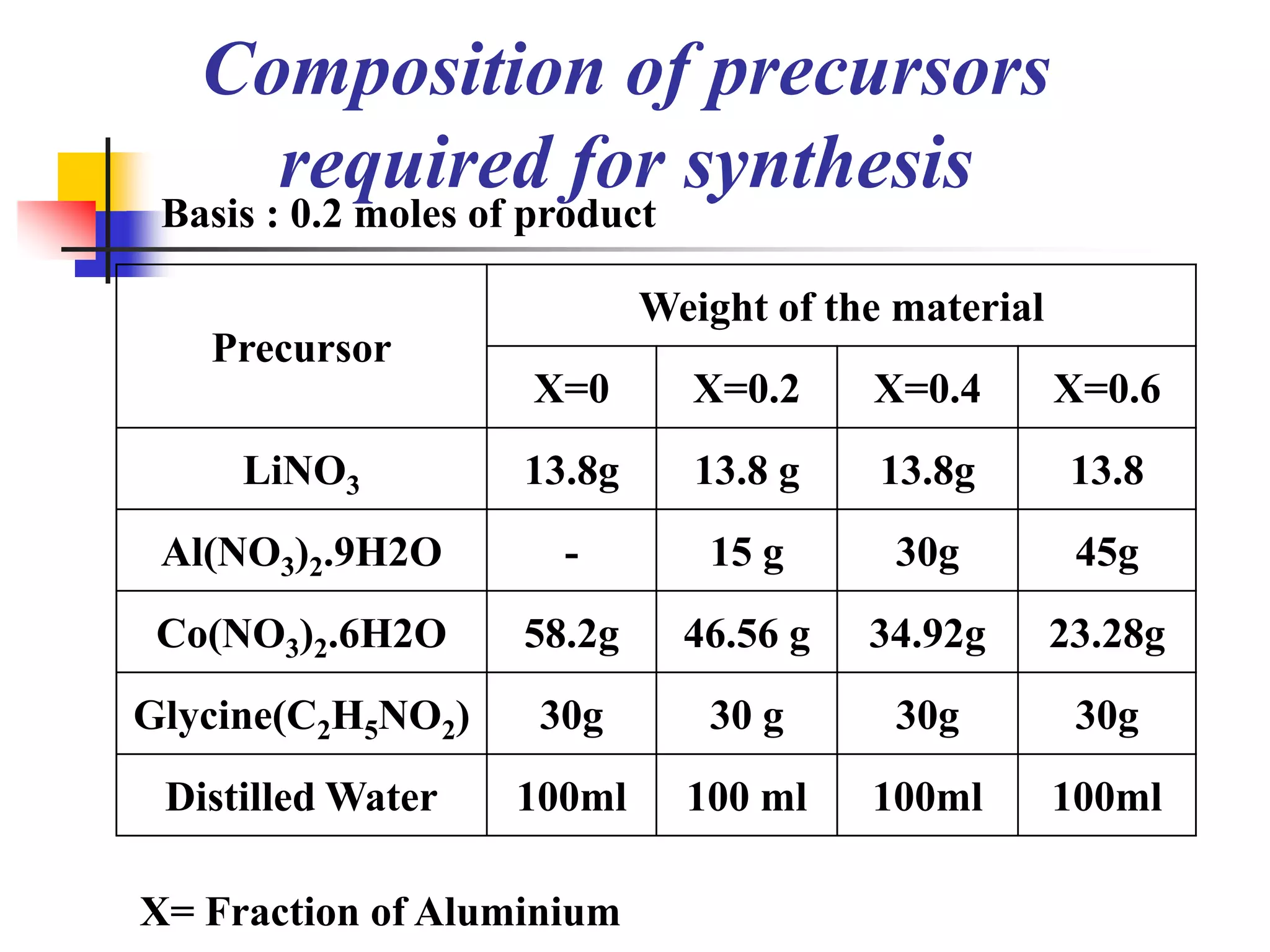
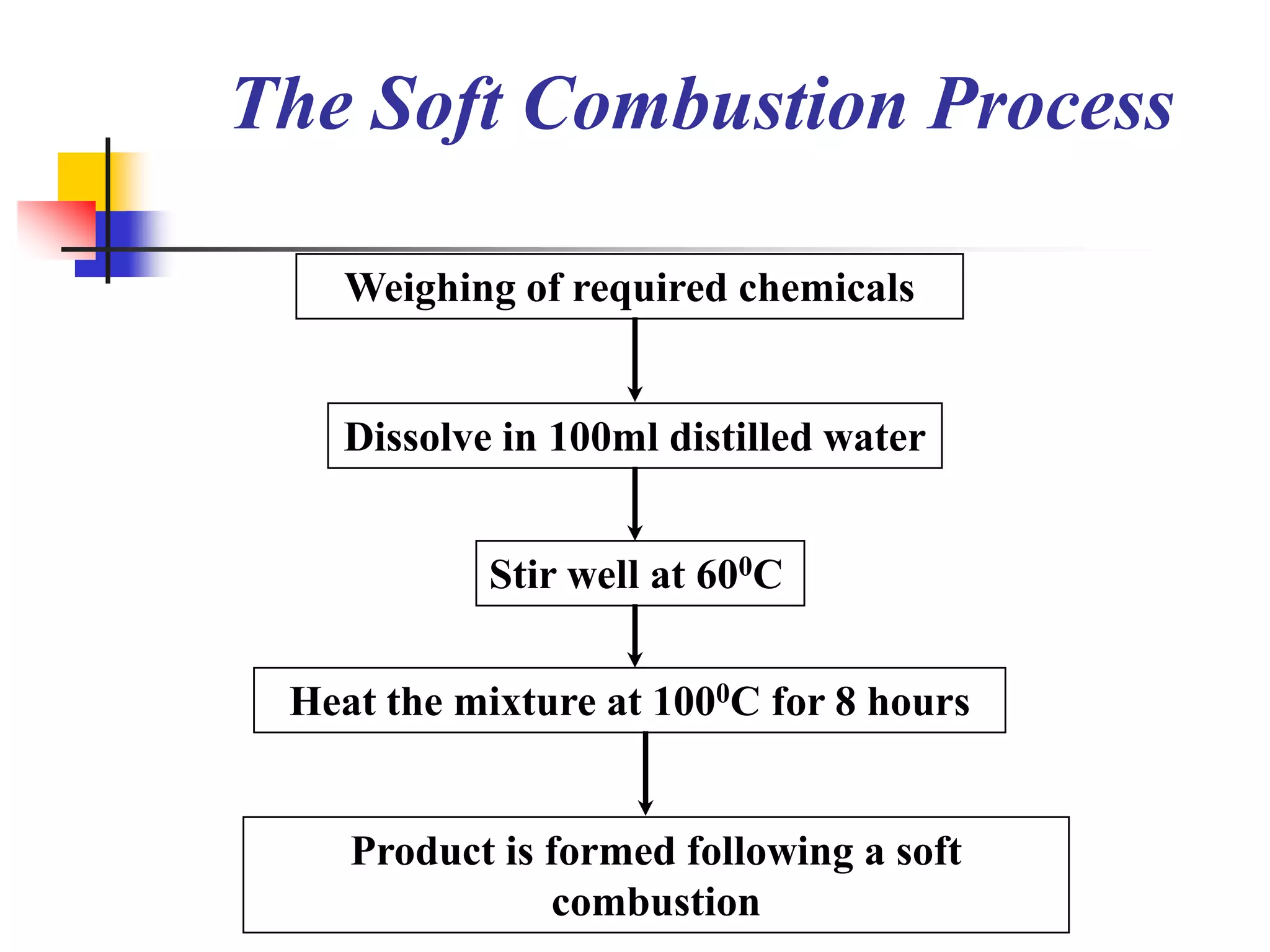
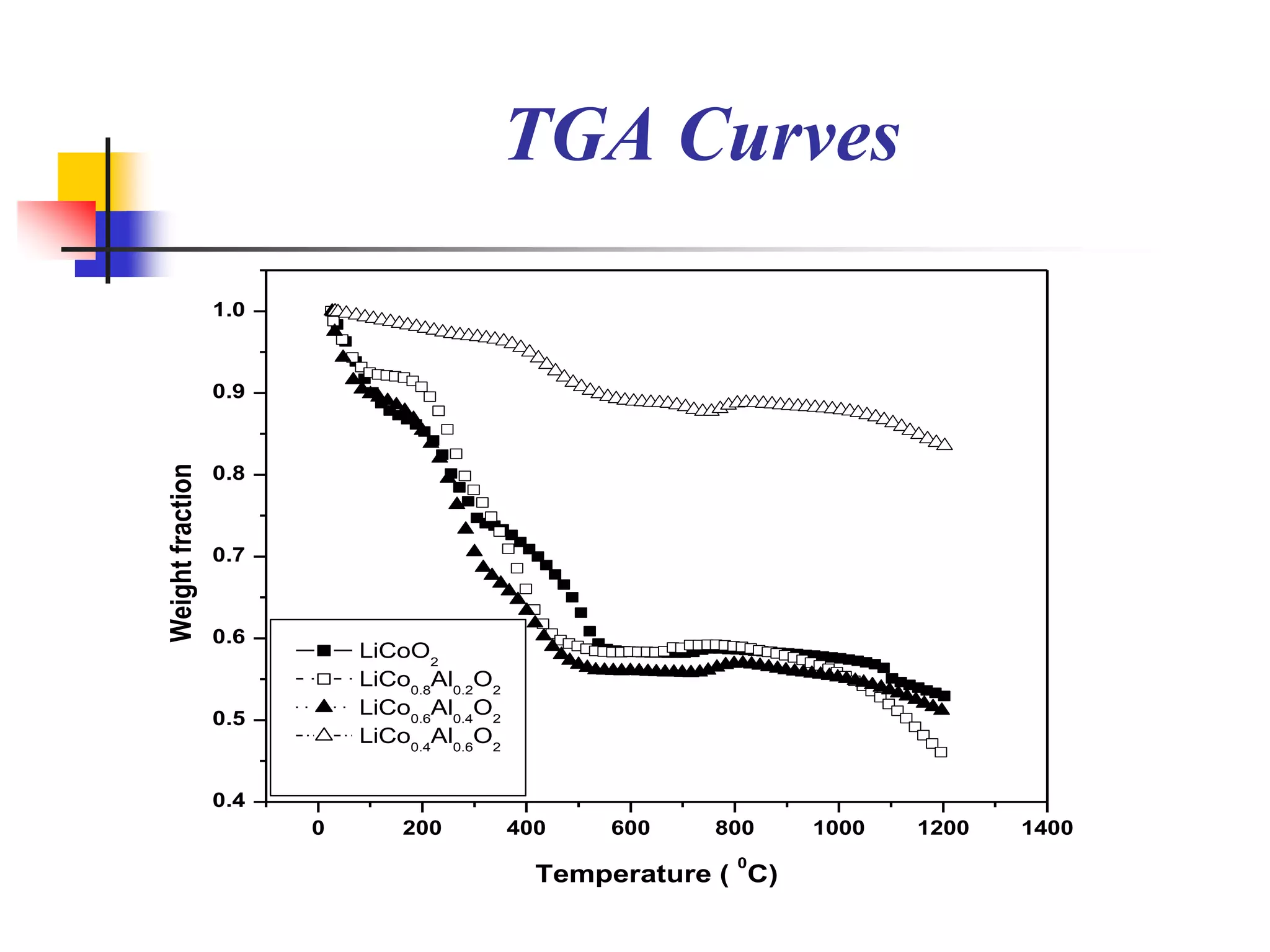
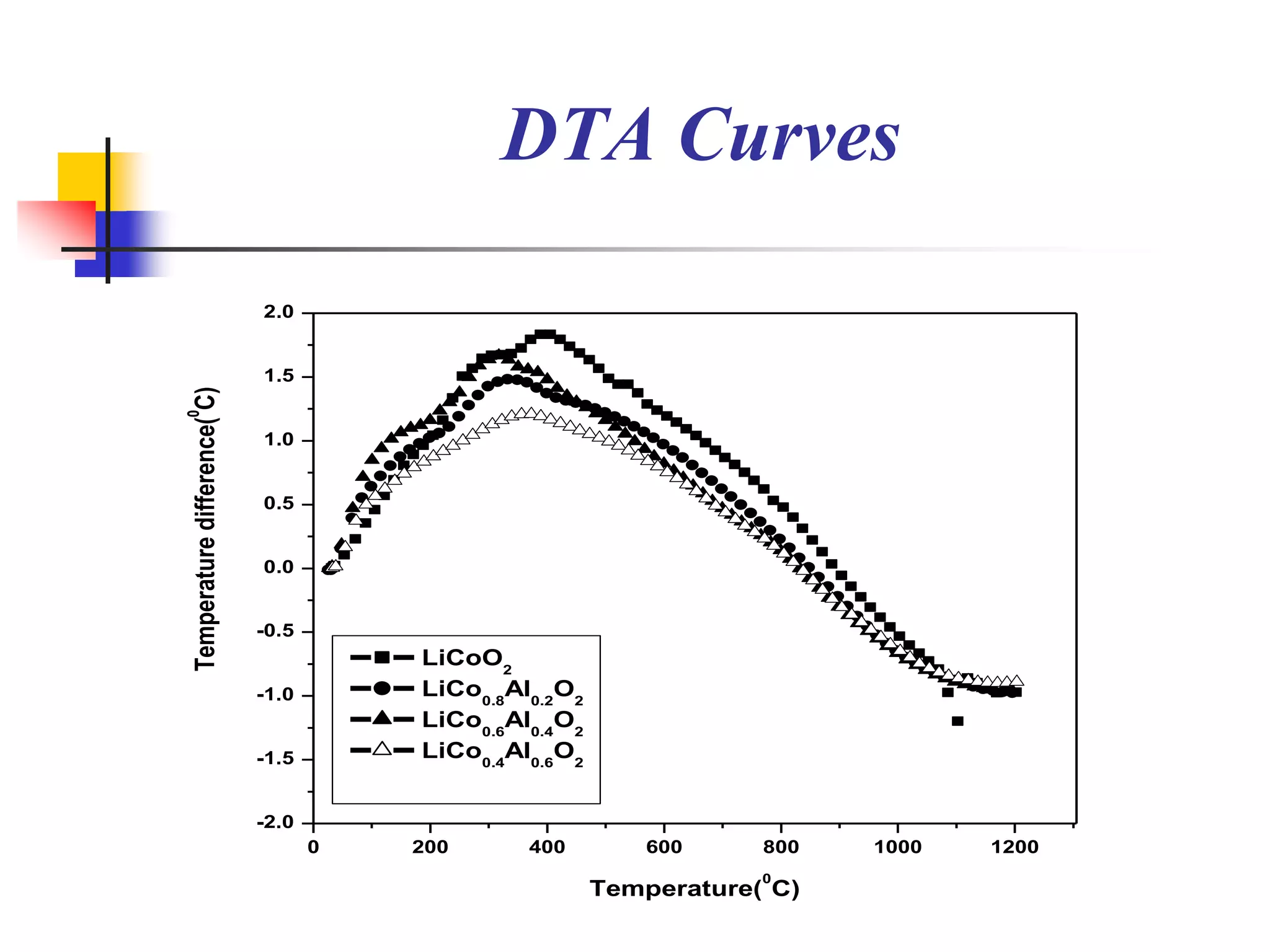
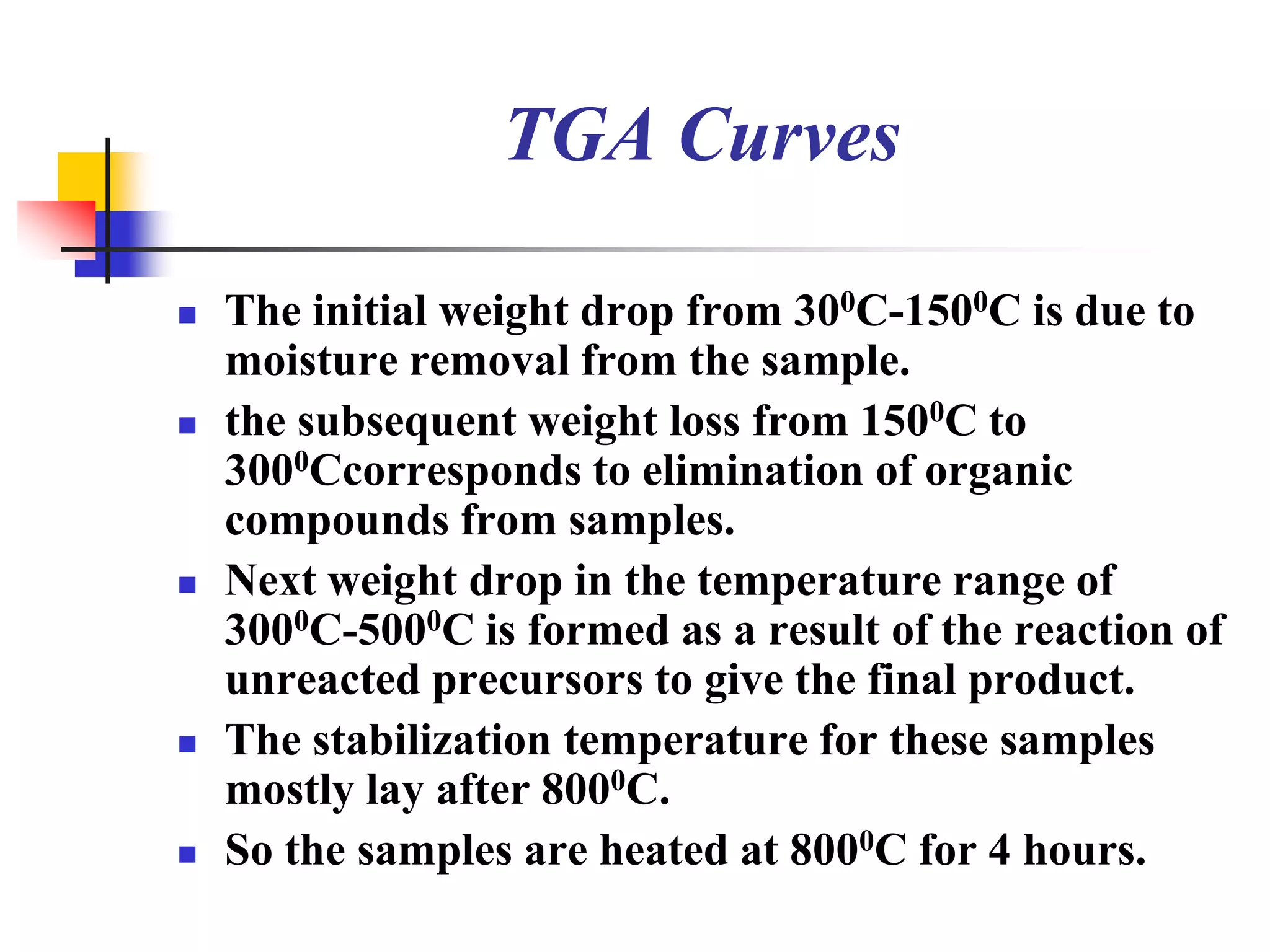
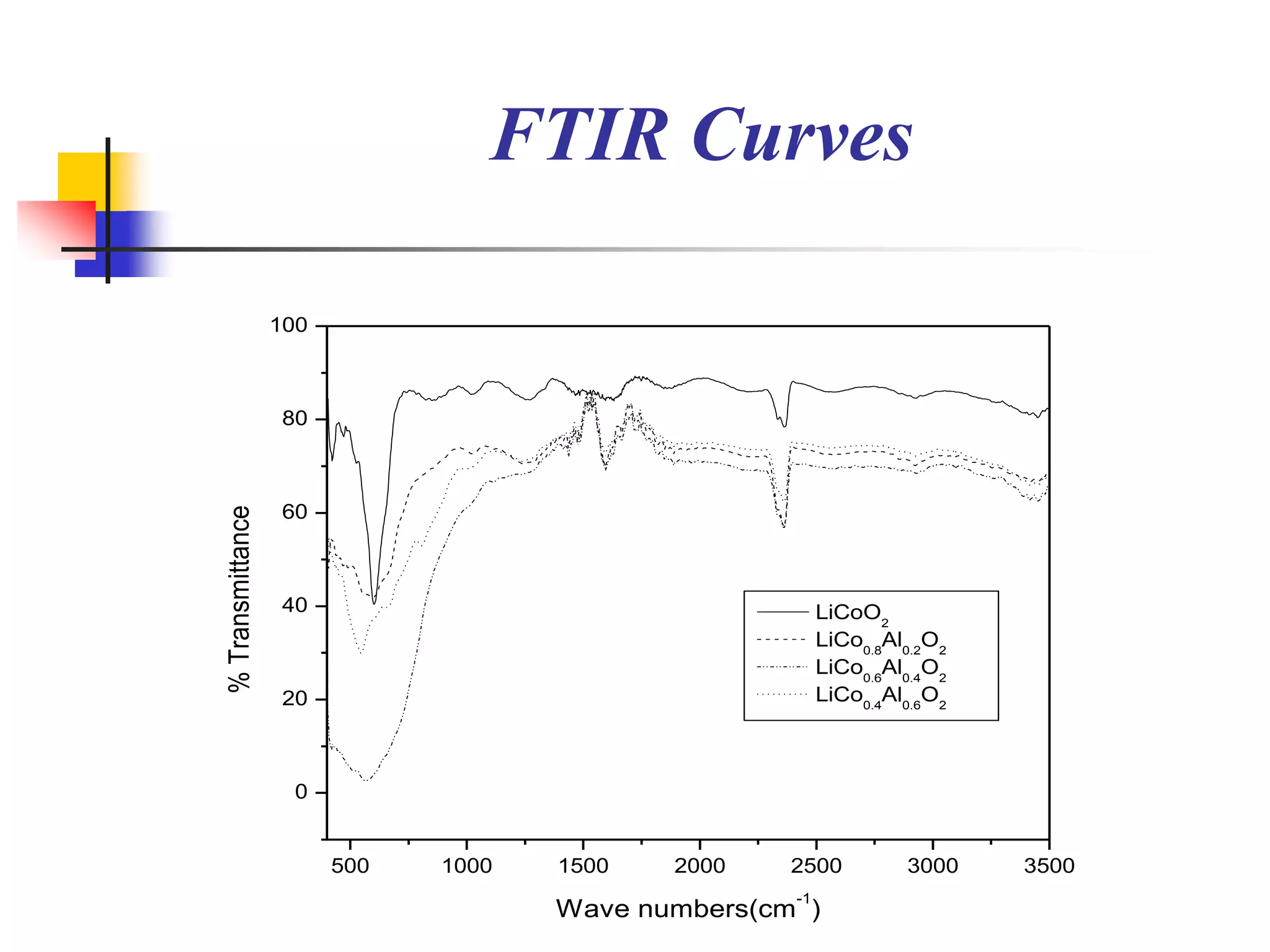
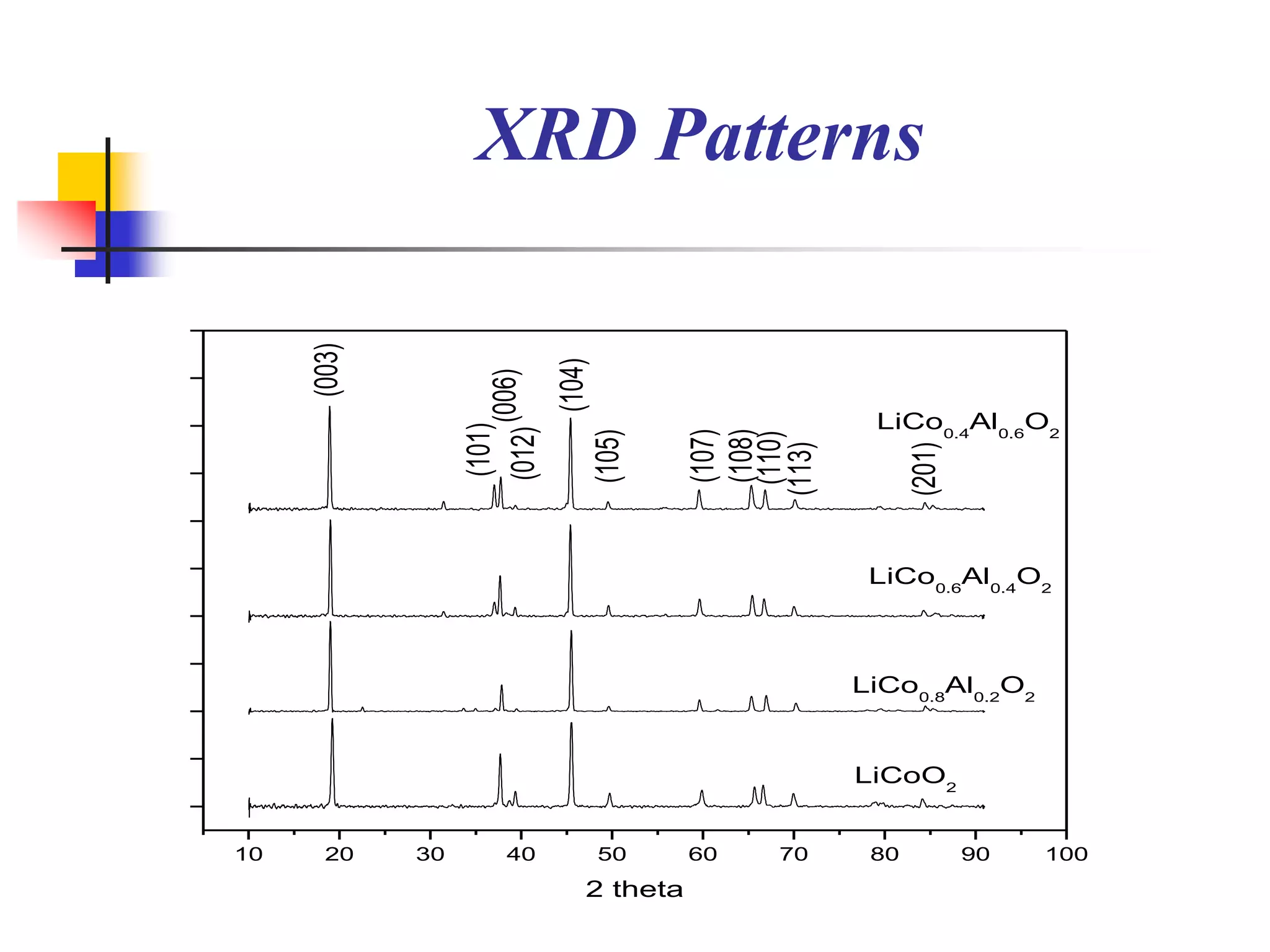
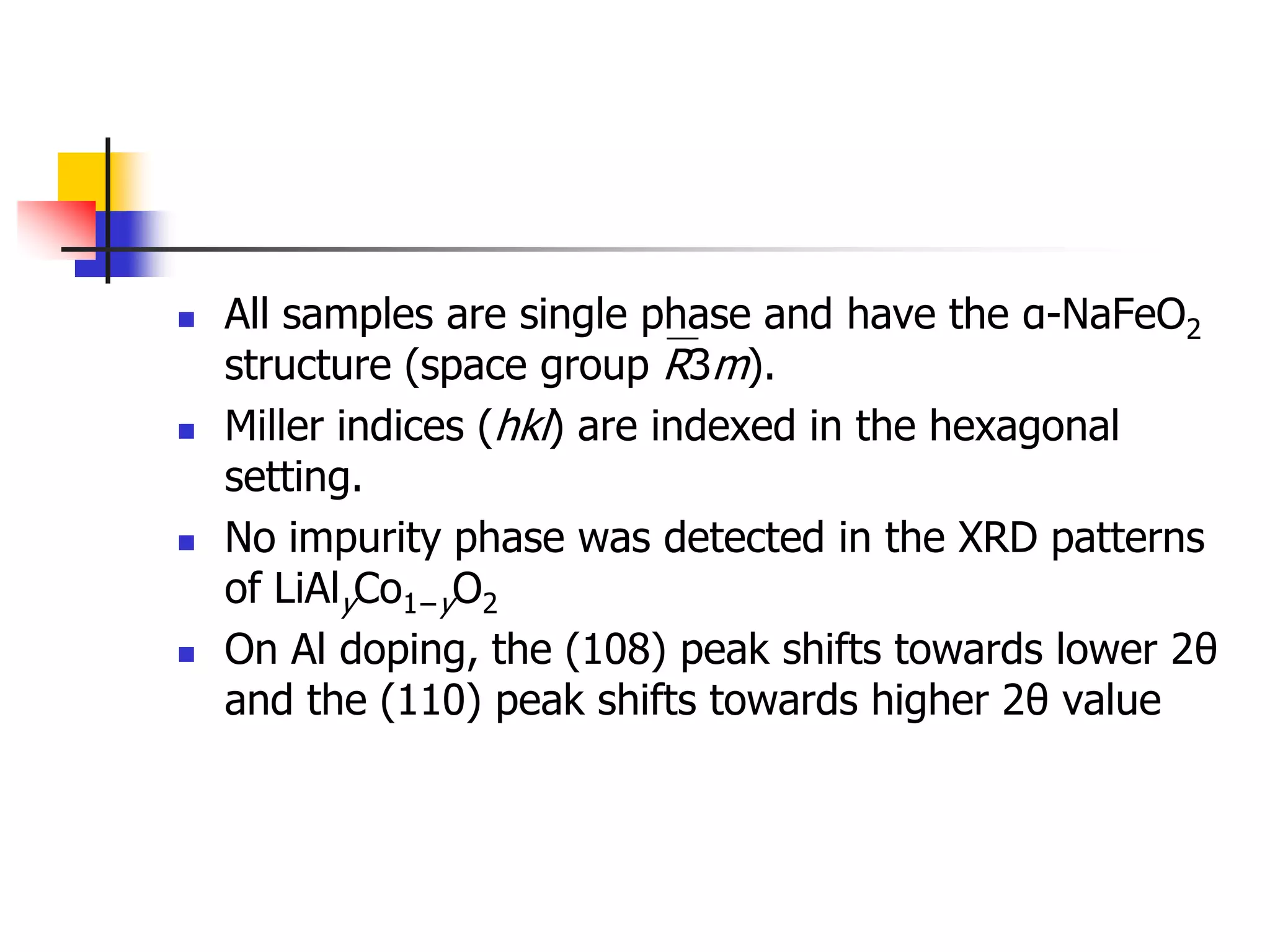
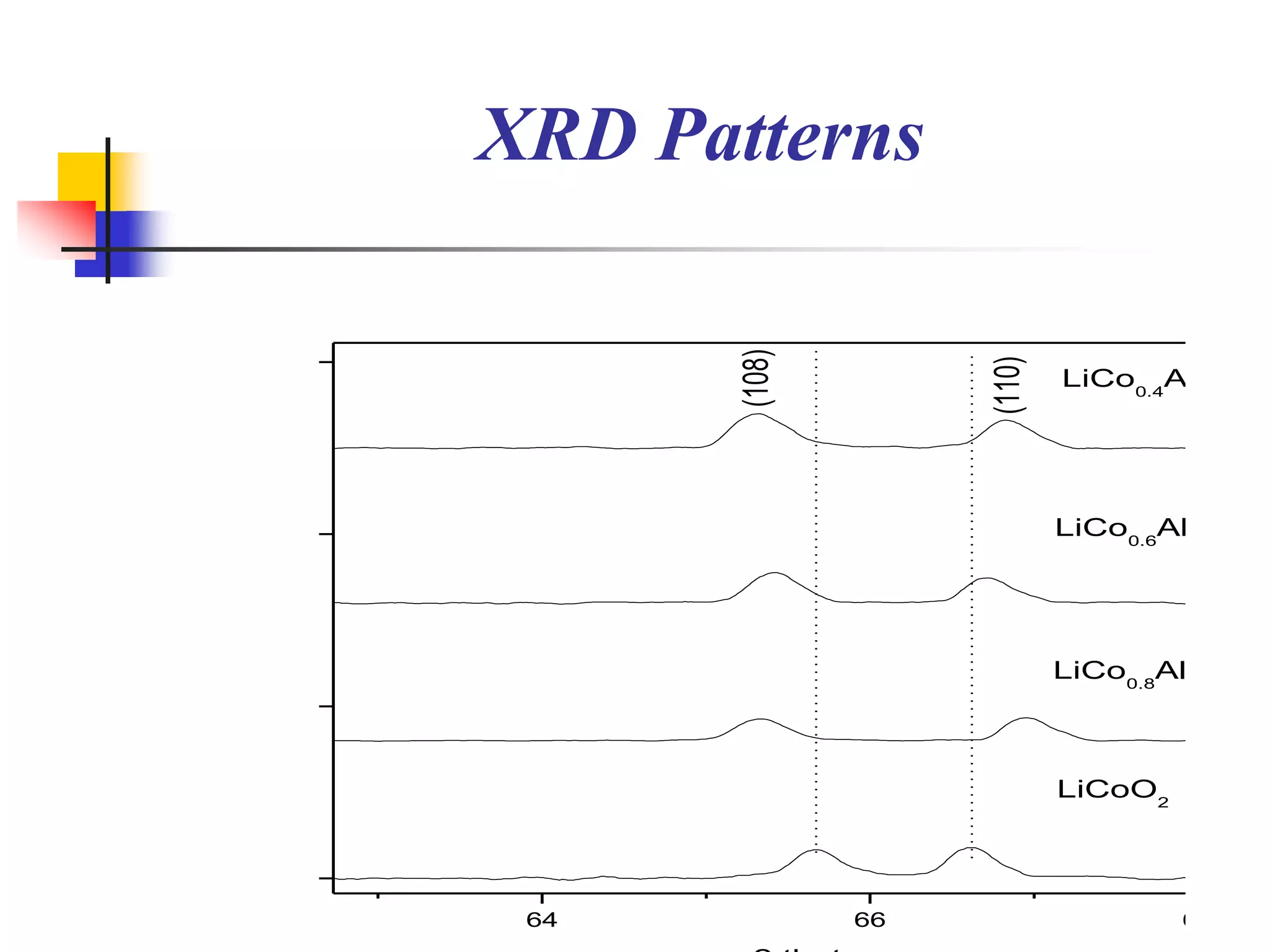
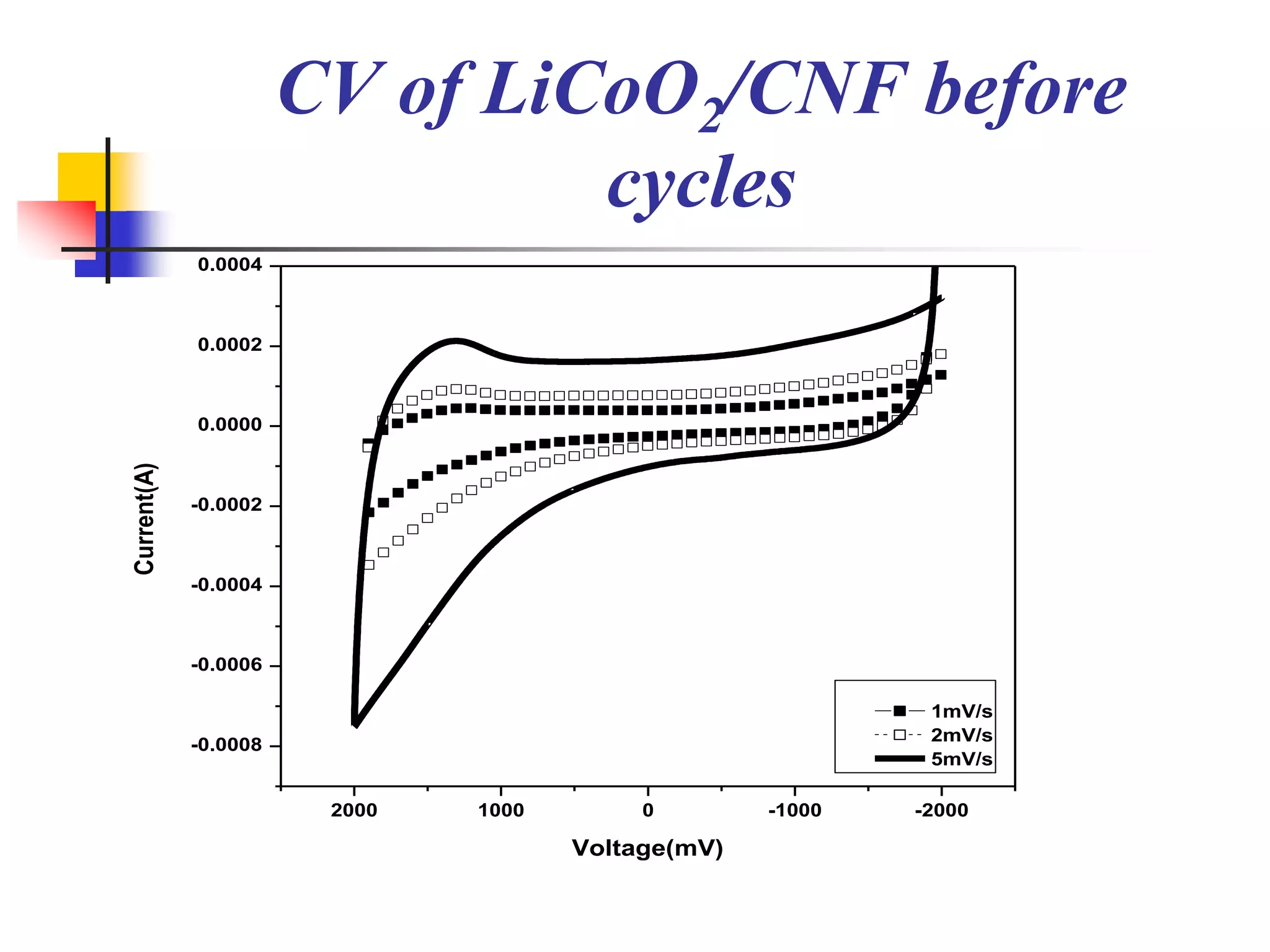
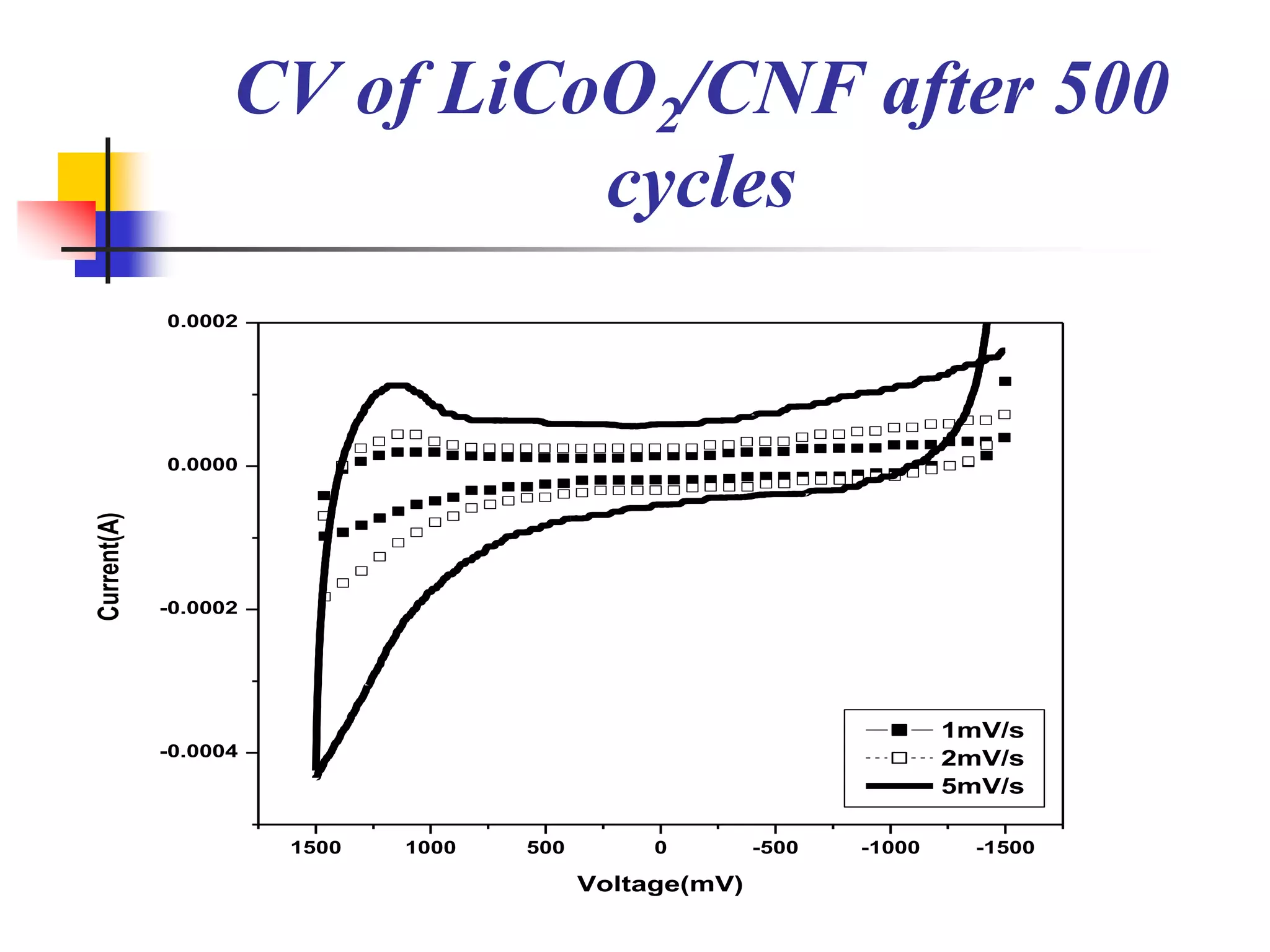
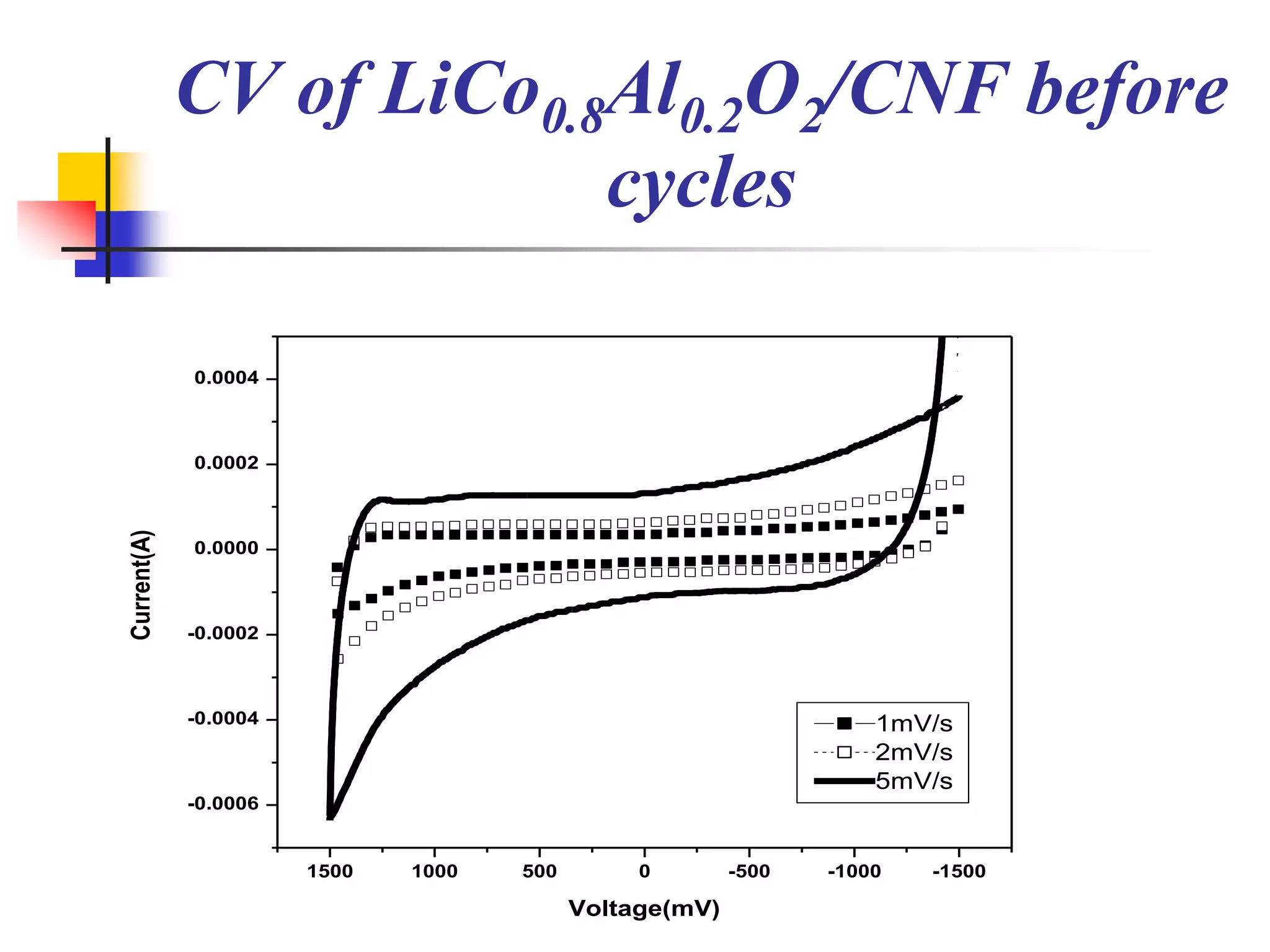
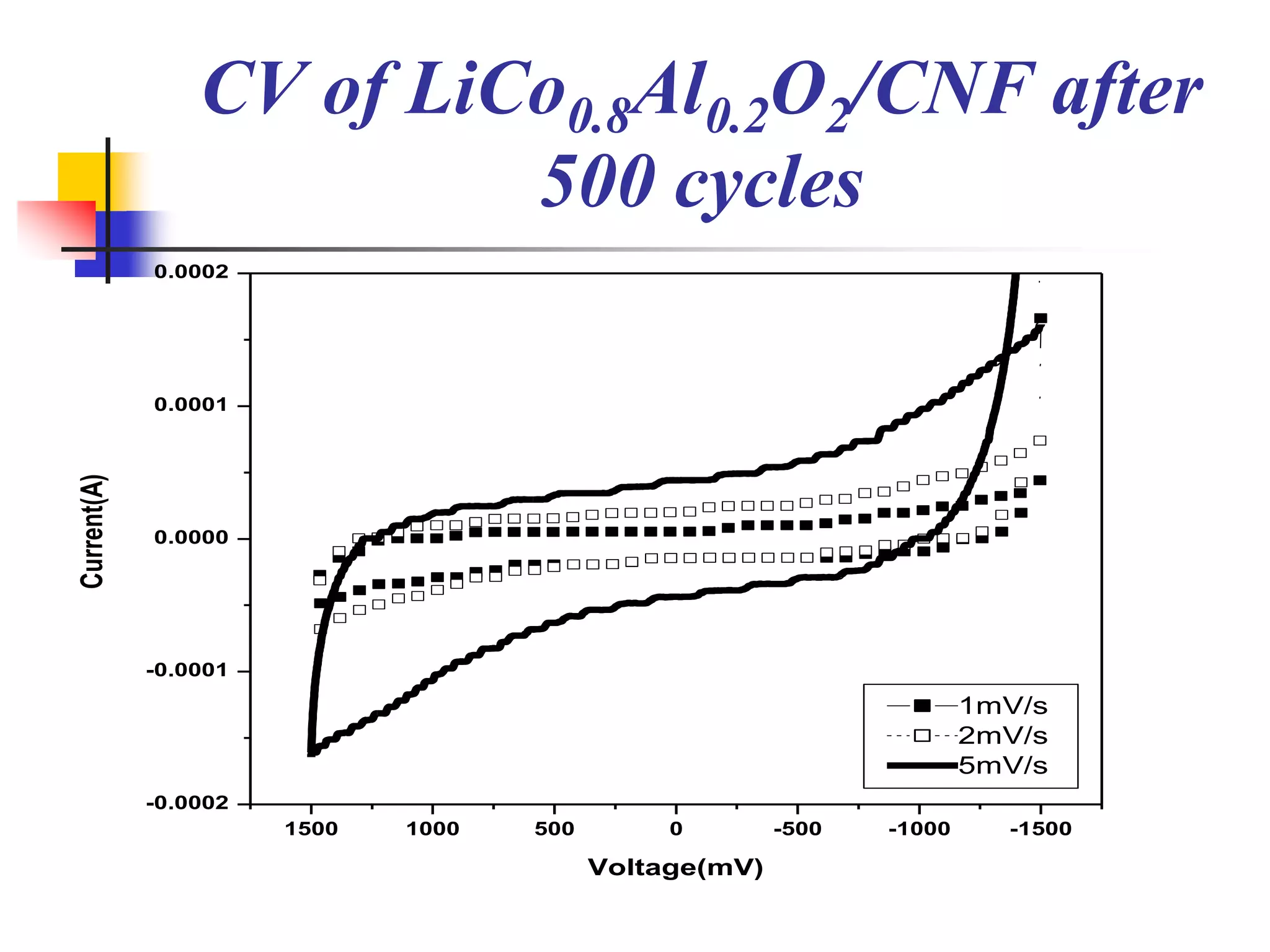
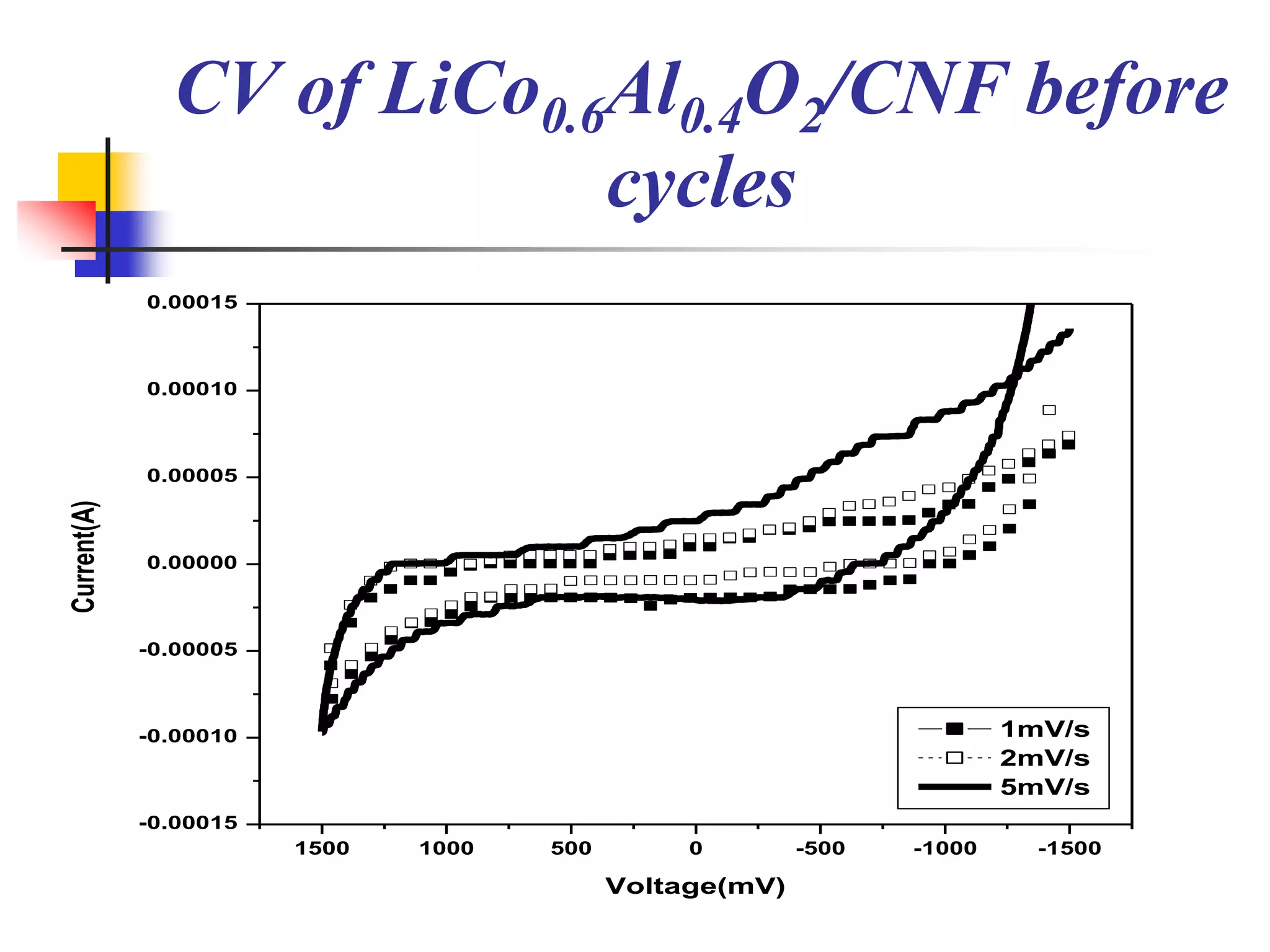
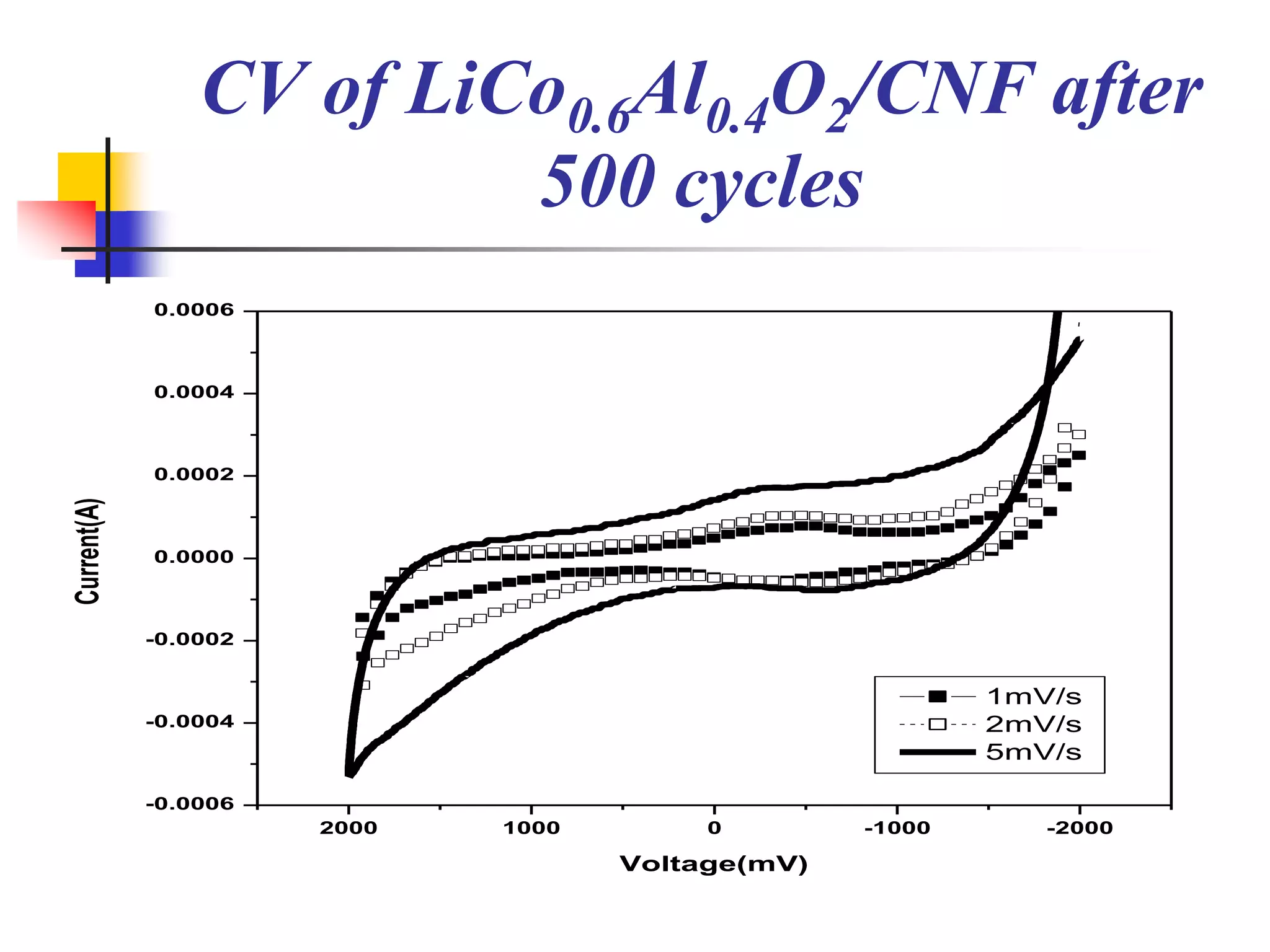
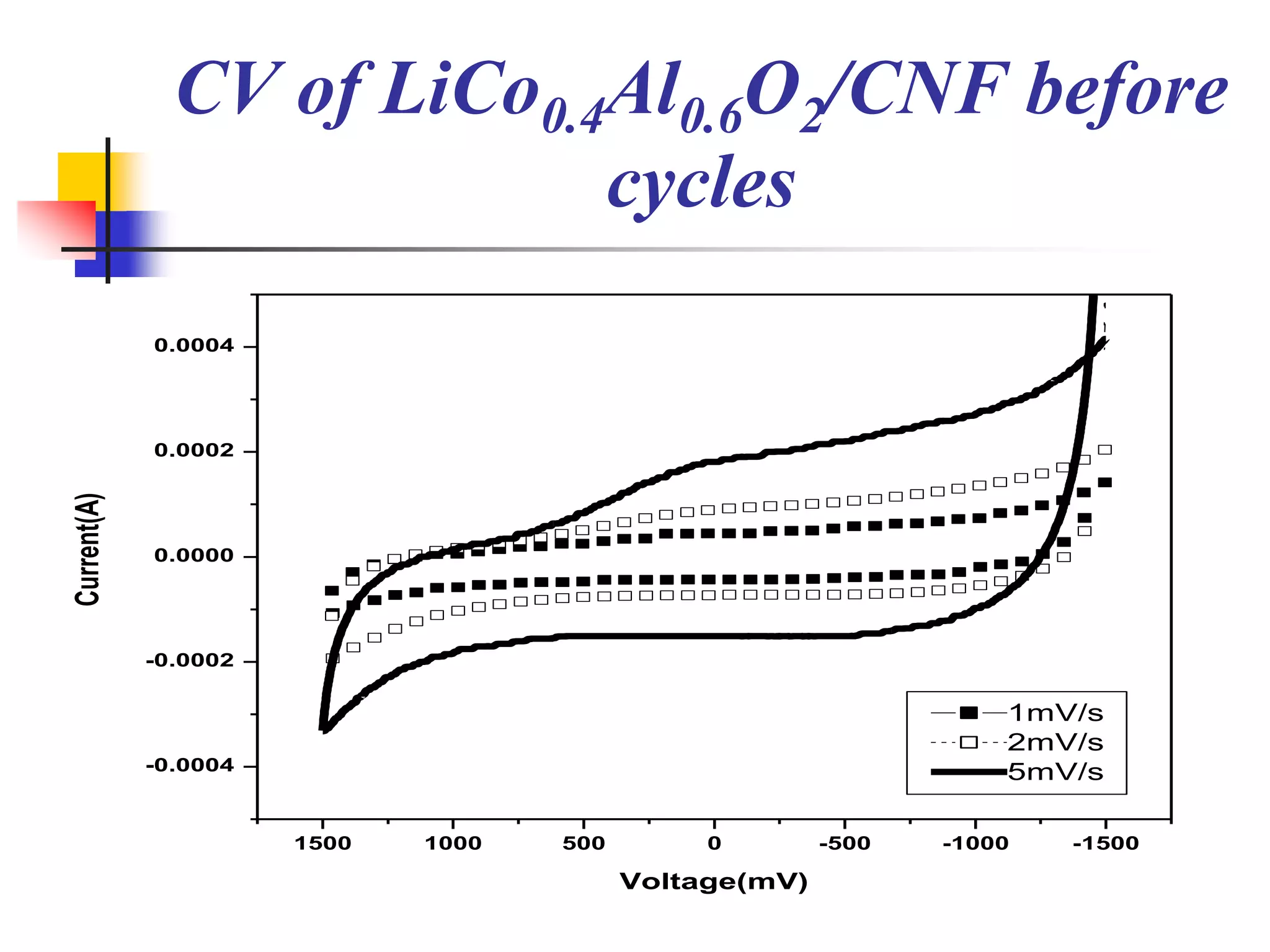
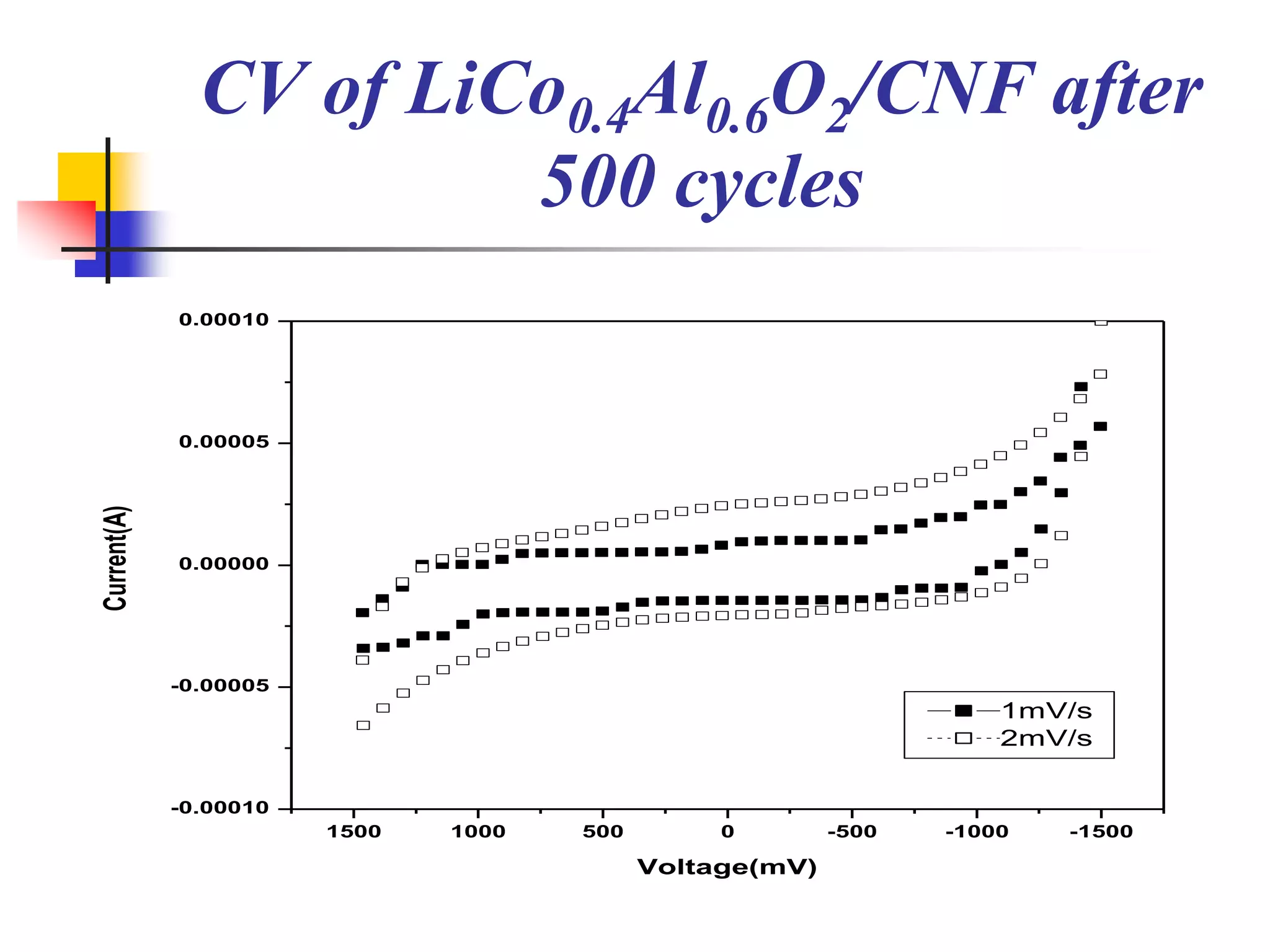
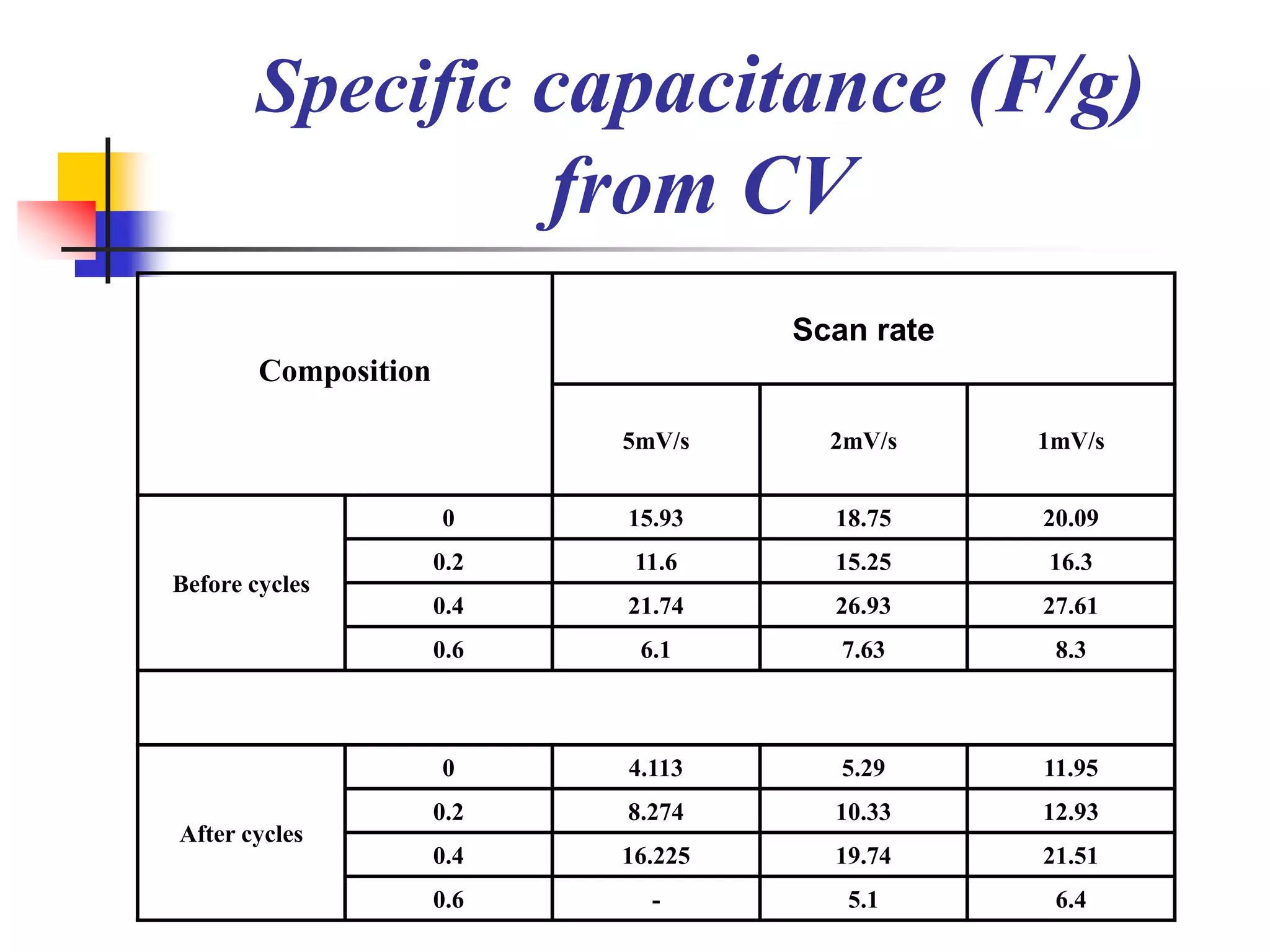
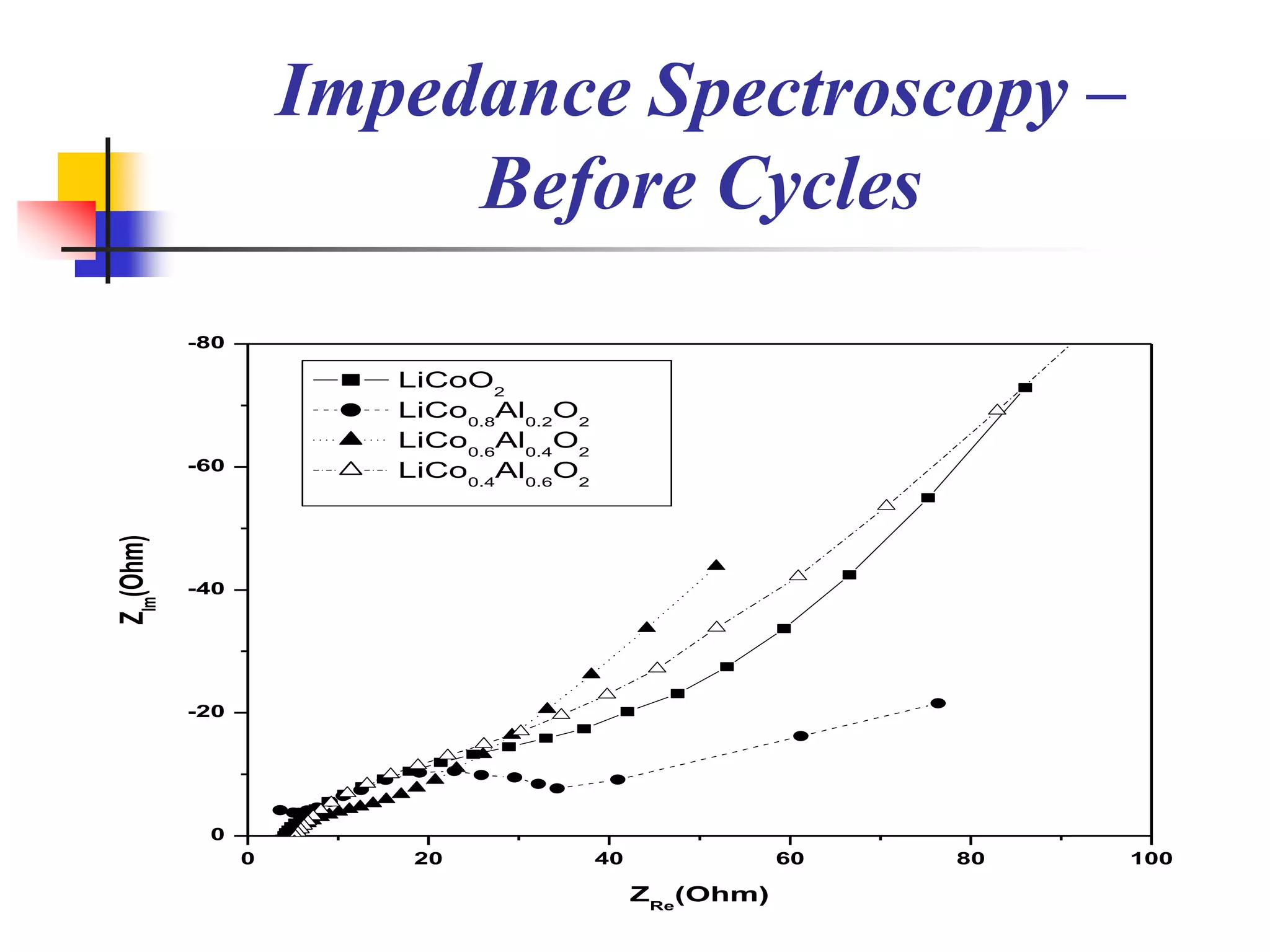
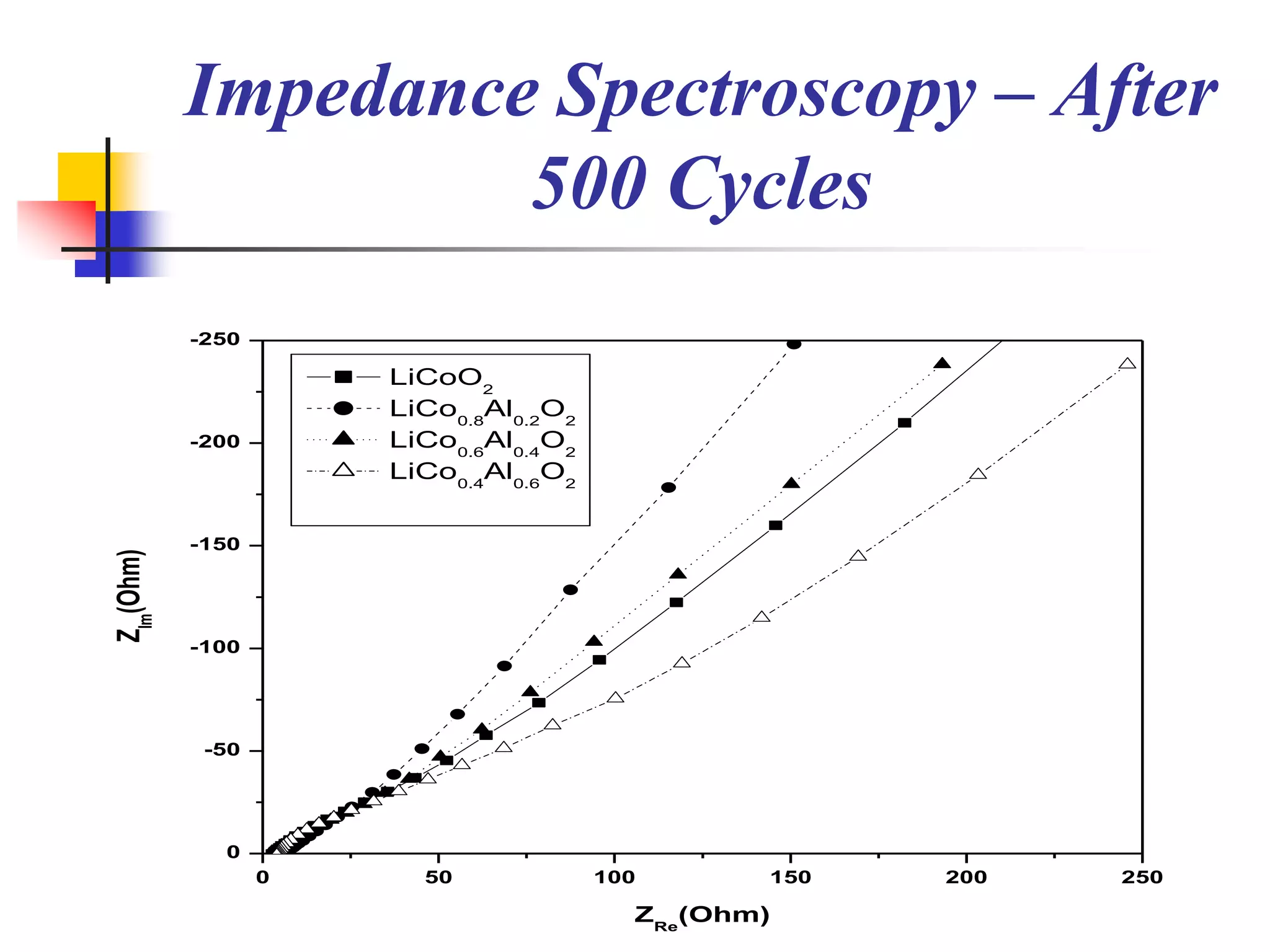
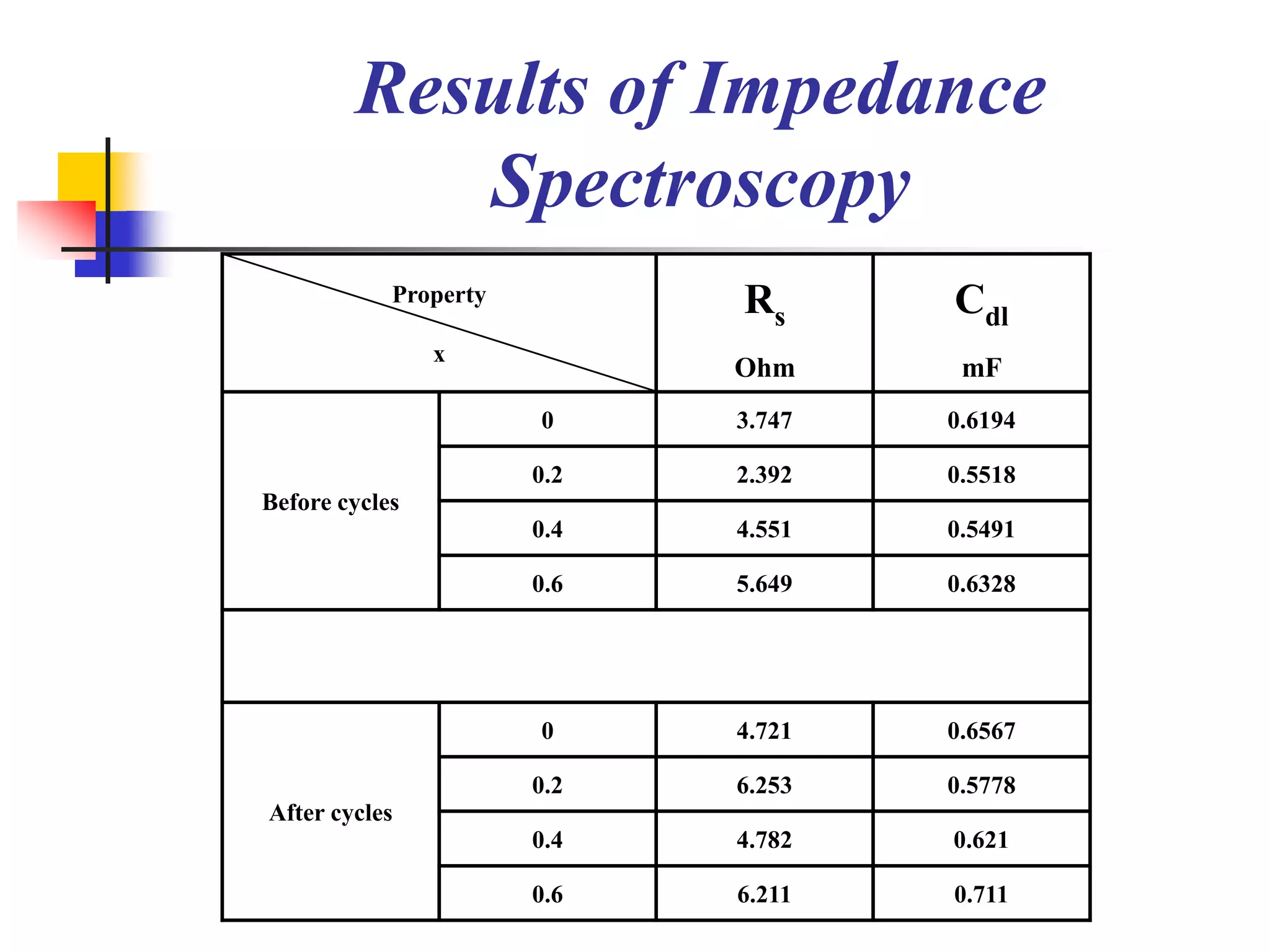
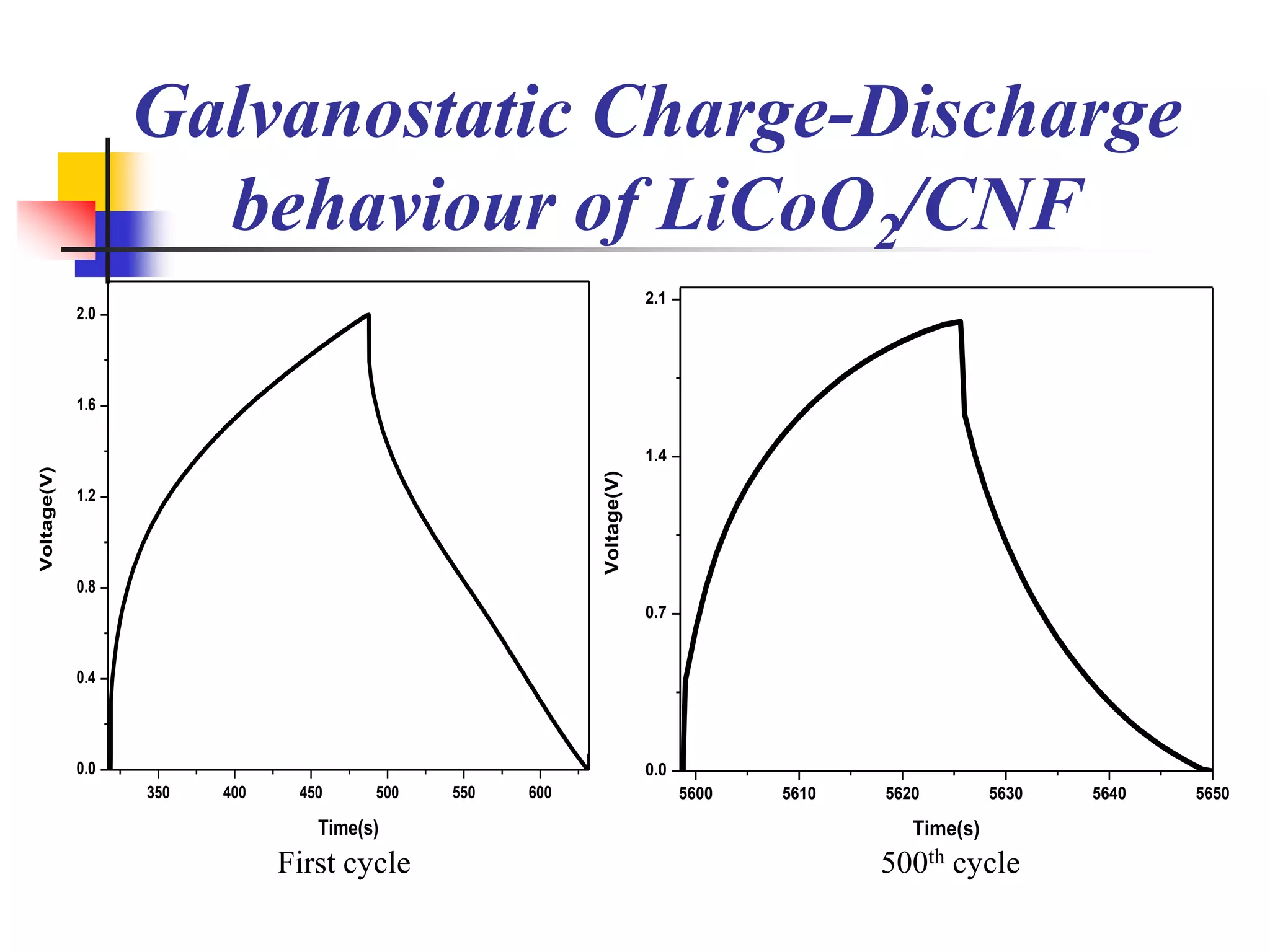
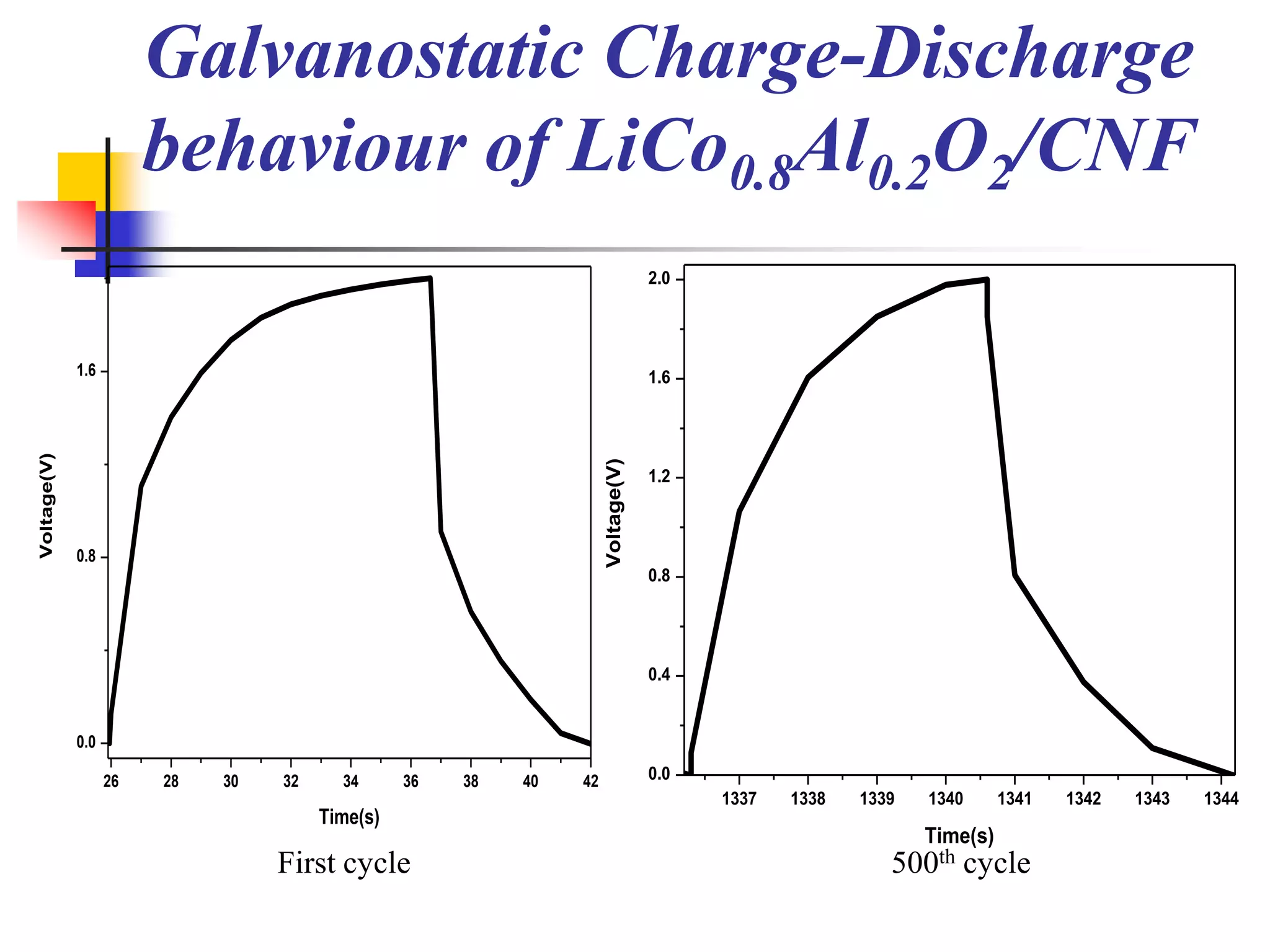
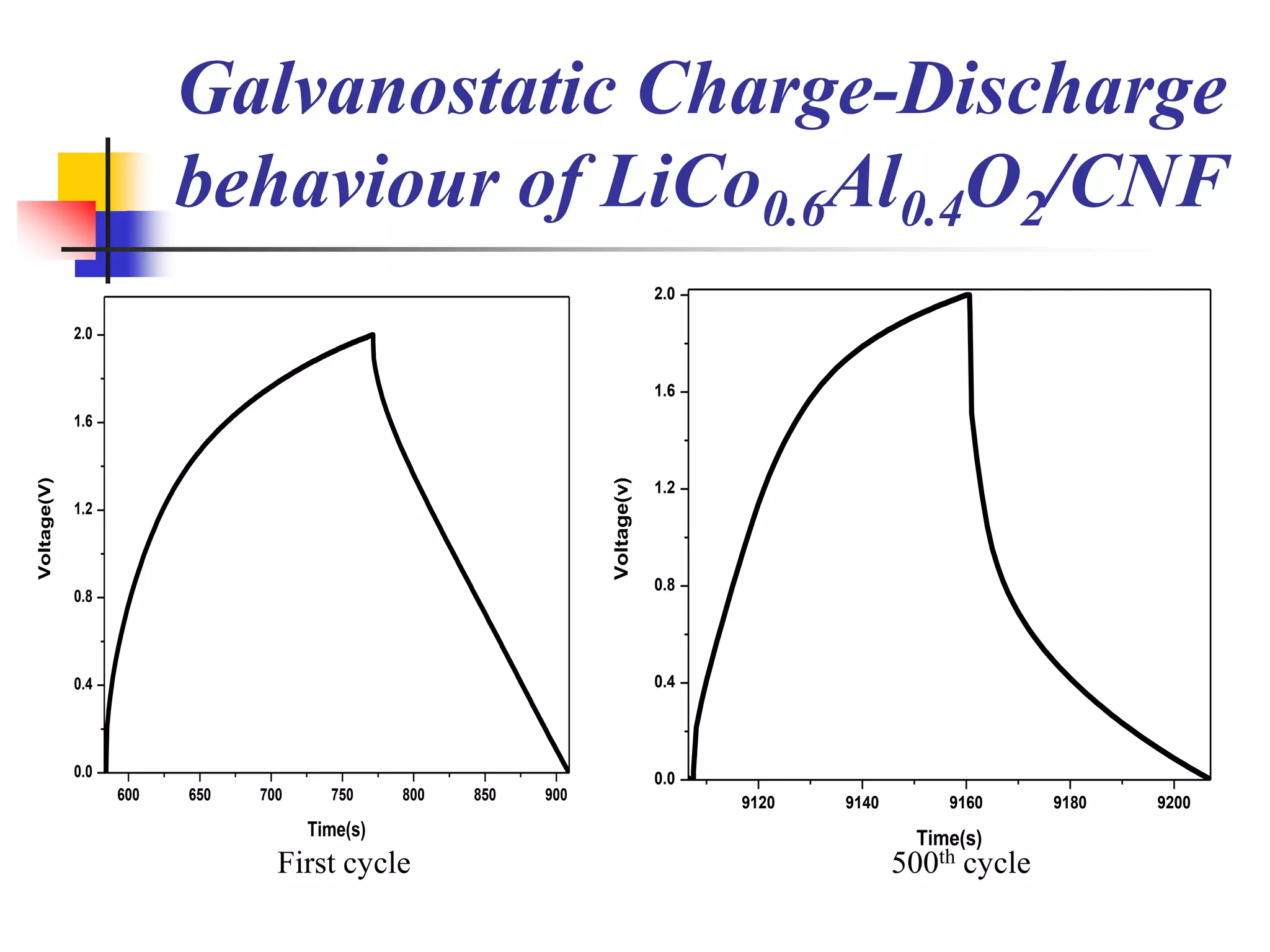
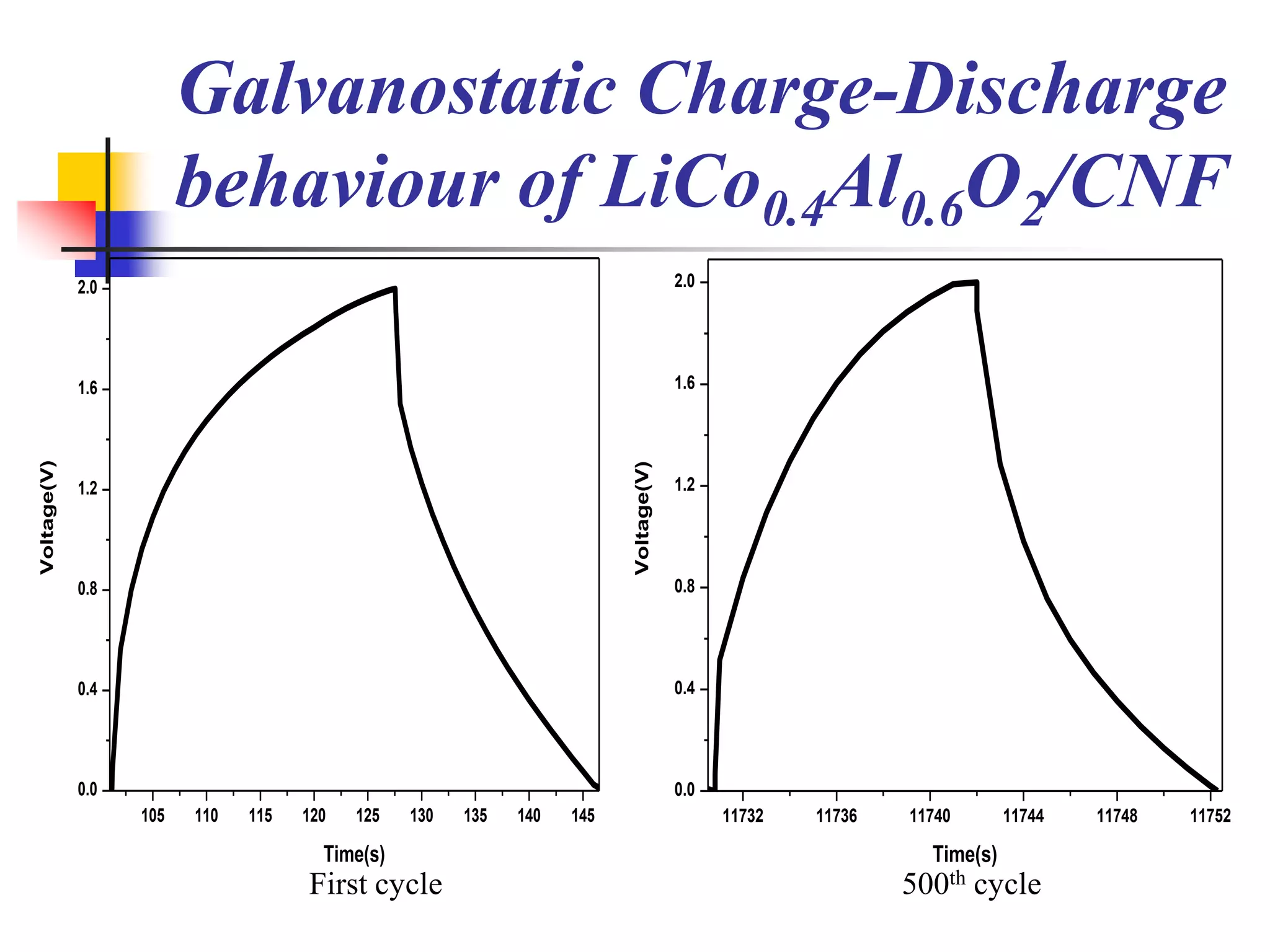
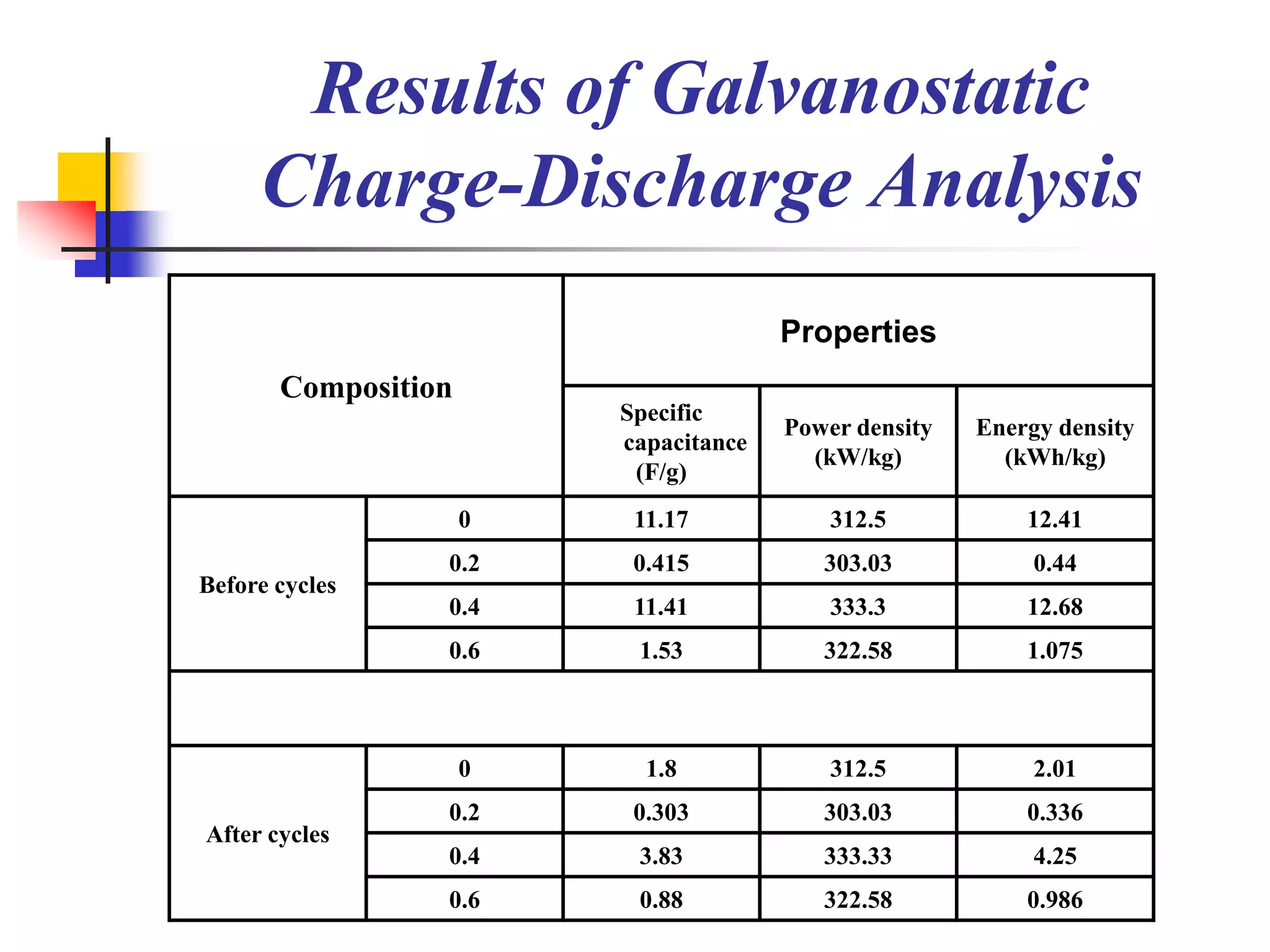
The document describes research on developing asymmetric hybrid supercapacitors based on lithium-ion intercalated compounds. Specifically, it discusses synthesizing cathode materials of pure LiCoO2 and LiCo1-xAlxO2 with varying amounts of aluminum doping (x=0, 0.2, 0.4, 0.6). The materials were characterized using techniques like thermal analysis, X-ray diffraction, and Fourier transform infrared spectroscopy. Electrochemical characterization of the materials was also performed through cyclic voltammetry and charge/discharge testing to evaluate their performance in supercapacitors.