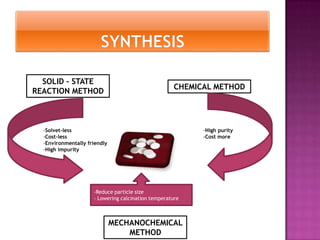
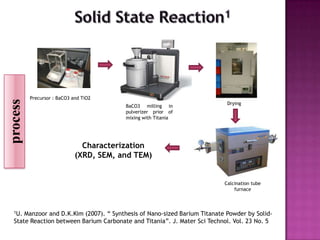
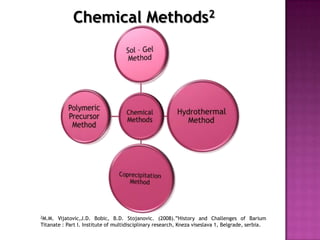
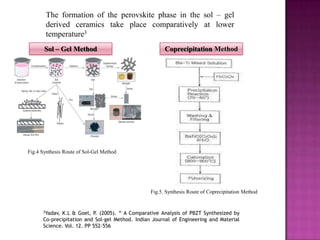
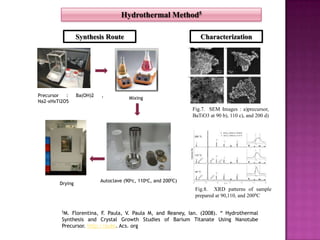
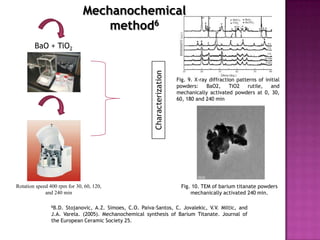
The document discusses several methods for synthesizing barium titanate including solid-state reaction, sol-gel, coprecipitation, polymeric precursor, hydrothermal, and mechanochemical methods. Each method has advantages and disadvantages in terms of cost, purity, particle size, and calcination temperature required. Characterization of synthesized barium titanate using techniques such as XRD, SEM, and TEM showed differences in microstructure and phase formation depending on the synthesis method and conditions used.