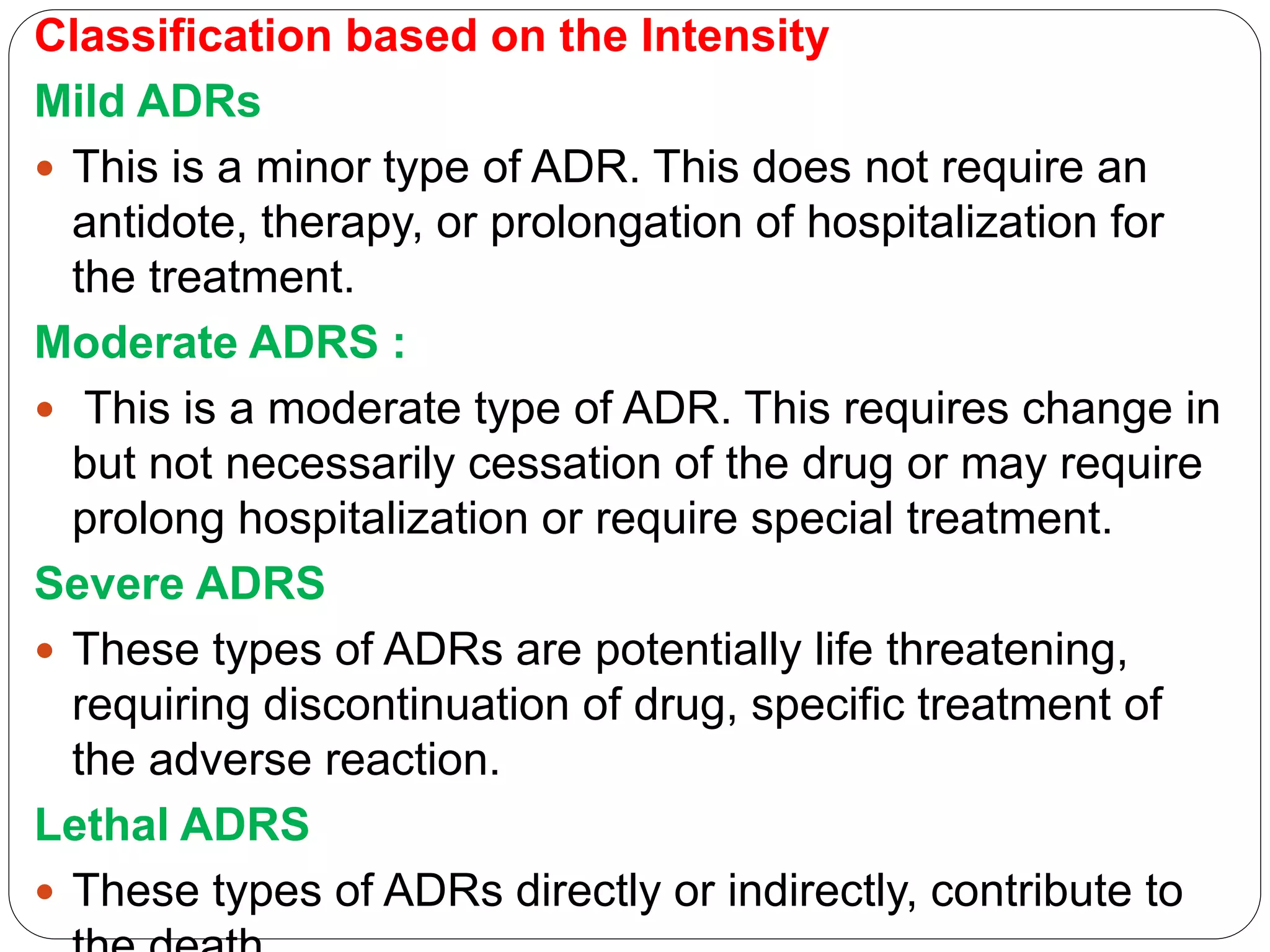
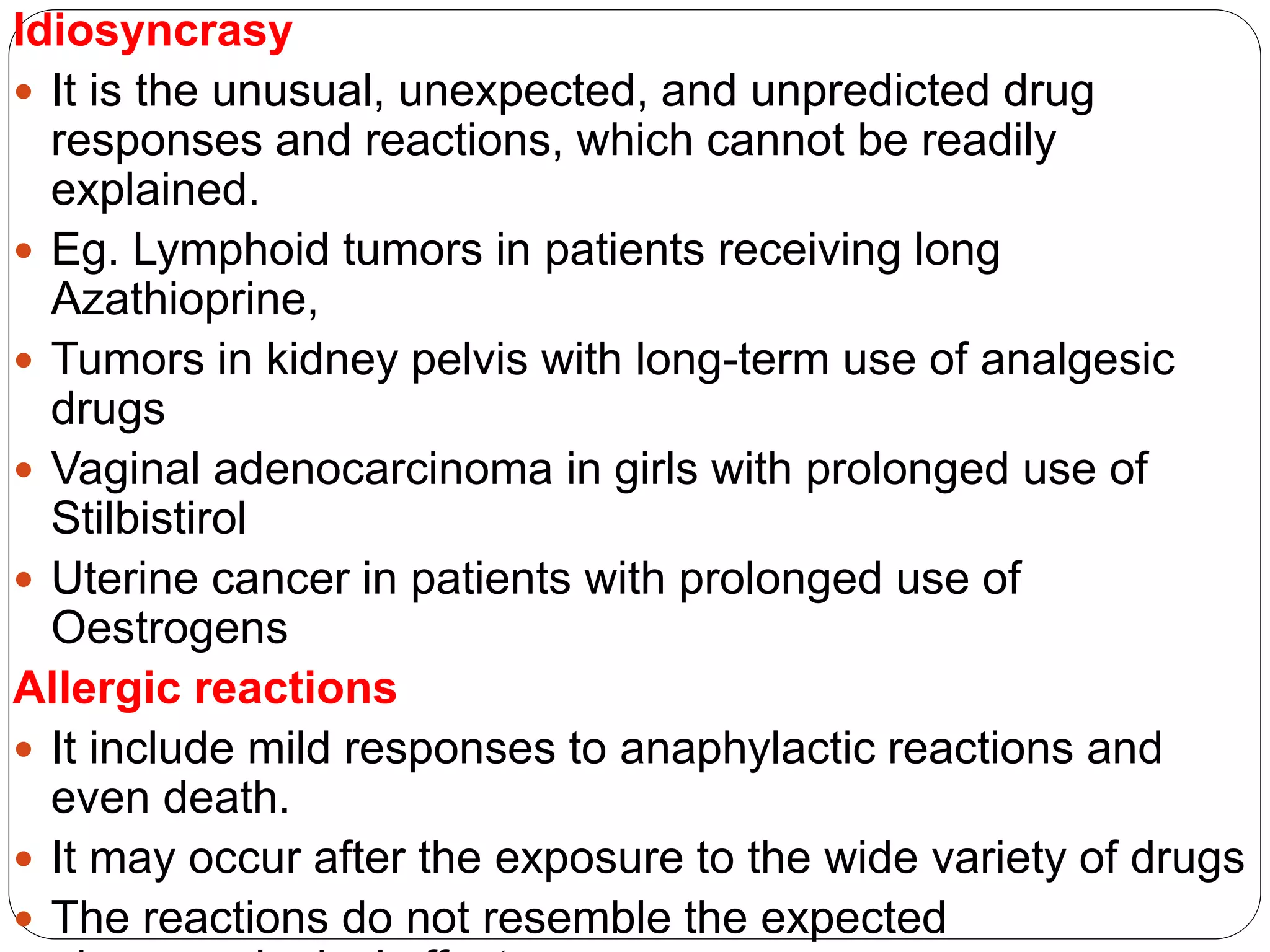
This document discusses adverse drug reactions (ADRs), including definitions from WHO and FDA, classification of ADRs as Type A or B reactions, factors affecting ADR incidence and severity, and methods for detecting and monitoring ADRs. It provides details on the national pharmacovigilance program in Nepal and the role pharmacists play in ADR monitoring and pharmacovigilance.