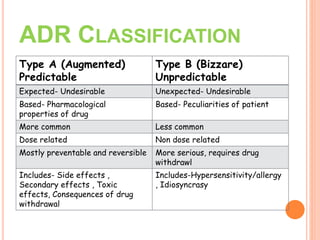
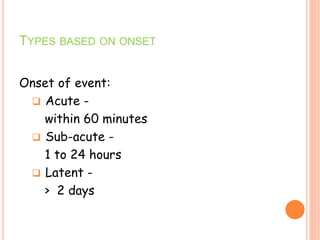
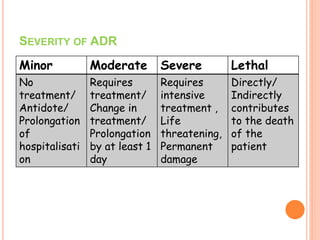
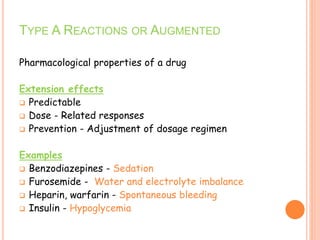
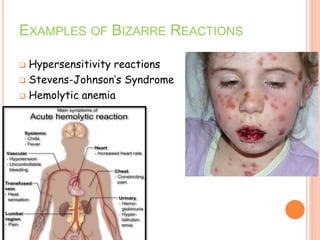
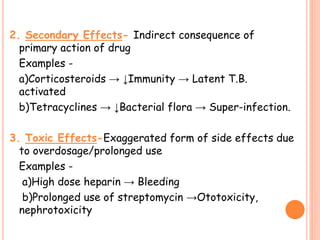
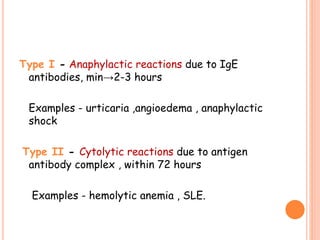
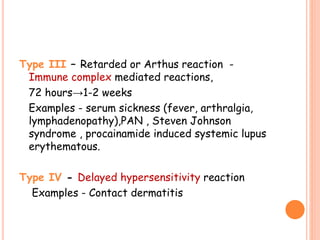
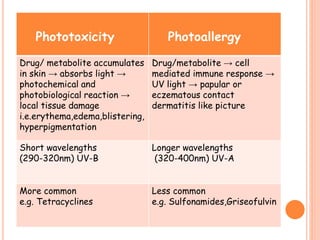
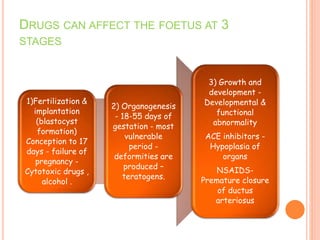
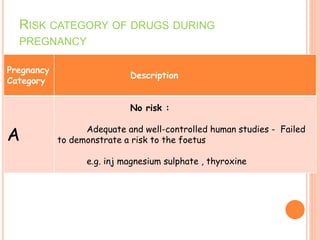
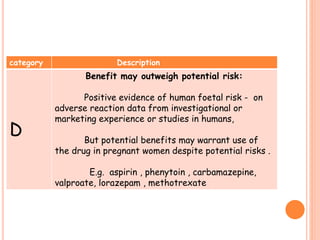
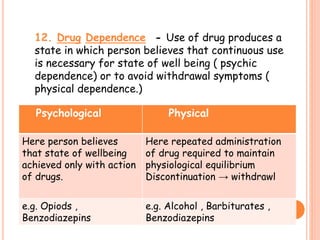
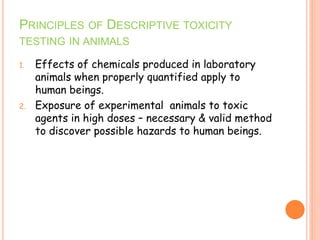
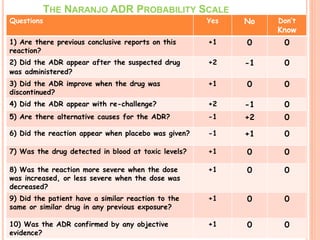
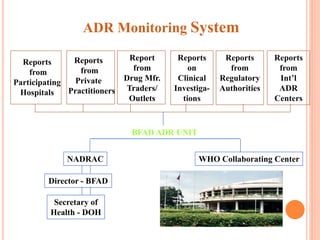
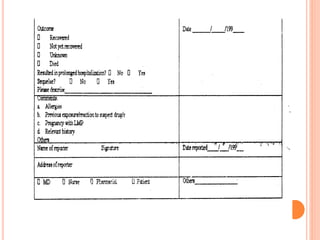
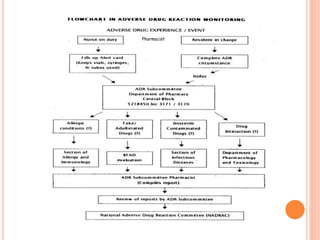
This document discusses adverse drug reactions (ADRs). It defines ADRs and provides statistics on their frequency and impact. It discusses various factors that can influence ADRs, including patient characteristics like age and genetics. It also discusses drug properties and interactions that can lead to ADRs. The document classifies ADRs into types A-F based on mechanisms and timing. It provides many examples of common and serious ADRs to illustrate different types. The document emphasizes the importance of pharmacovigilance in monitoring and preventing ADRs.