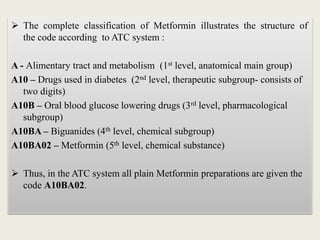
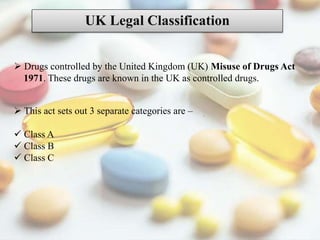
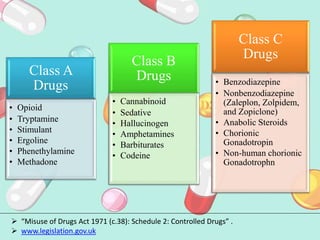
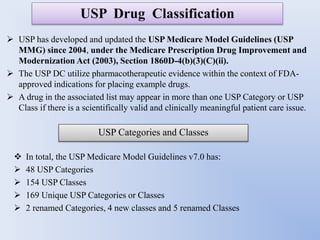
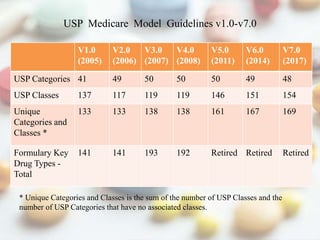
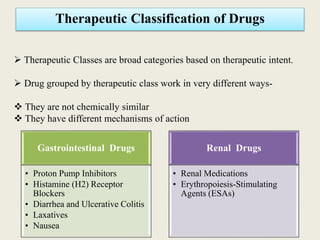
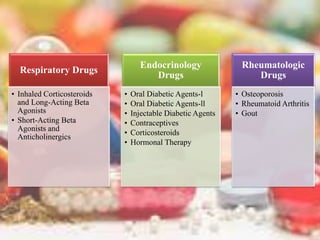
This document discusses various methods of classifying drugs. It describes the Anatomical Therapeutic Chemical (ATC) system used by the WHO to categorize drugs based on organ system, therapeutic effects, and chemical properties. It also outlines the US Drug Enforcement Administration's system of classifying drugs into 5 schedules based on their medical use, abuse potential, and dependence. Finally, it discusses pharmacological classification based on mechanism of action and therapeutic classification focused on therapeutic intent.