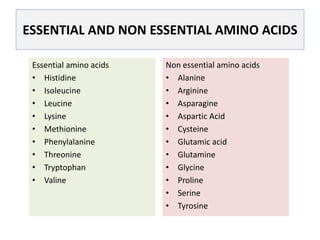
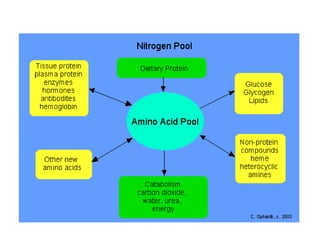
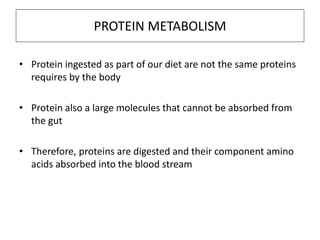
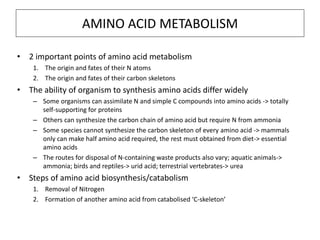
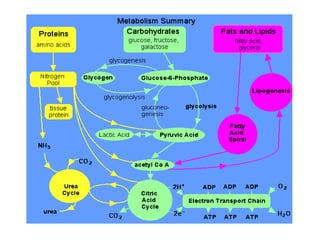
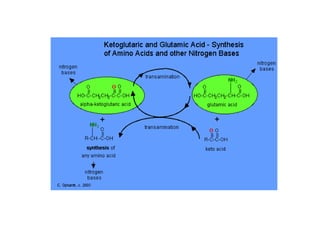
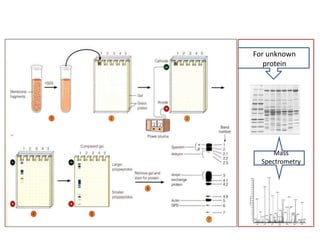
This document discusses protein metabolism, including essential and non-essential amino acids. Essential amino acids cannot be synthesized by the human body and must be obtained through diet. Non-essential amino acids can be synthesized from other amino acids or compounds. The document also outlines the processes of protein digestion, amino acid catabolism through transamination and the urea cycle, and analytical techniques for purifying and characterizing proteins like polyacrylamide gel electrophoresis and Edman degradation.