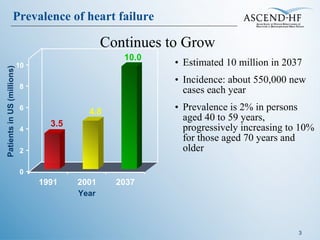
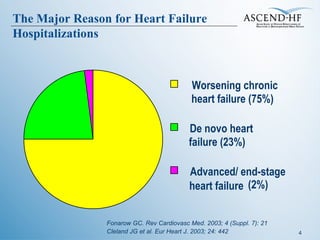
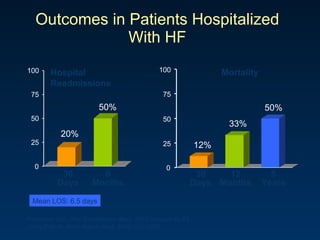
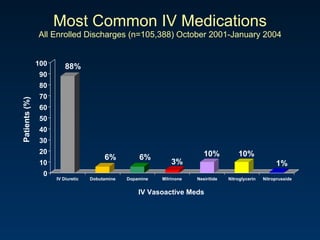
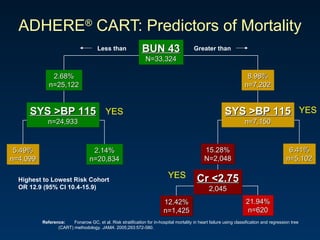
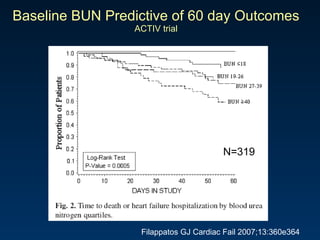
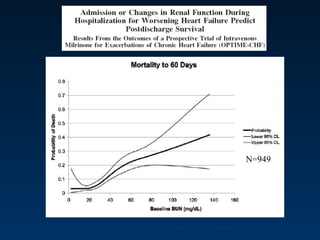
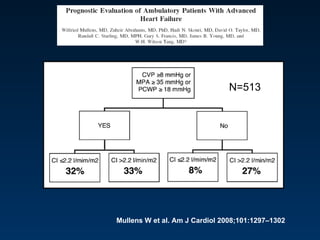
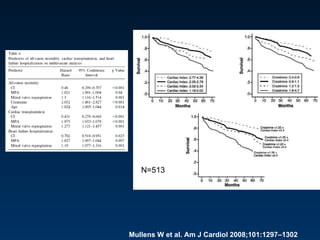
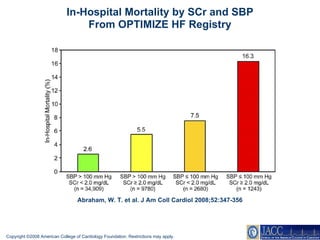
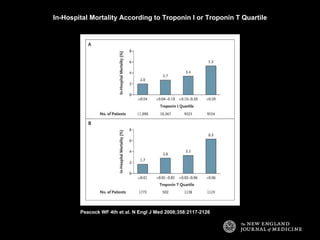
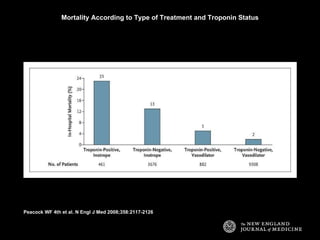
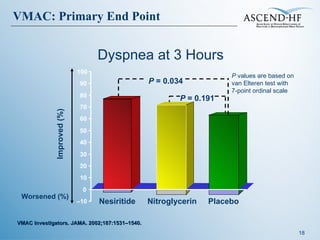
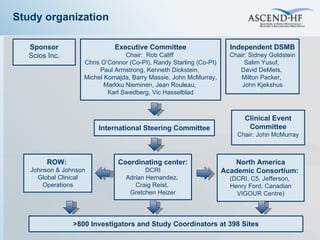
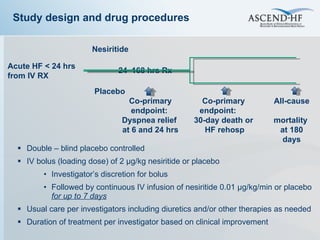
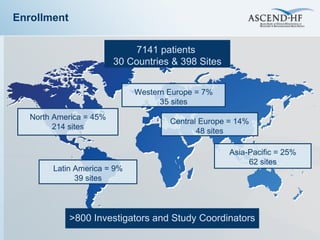
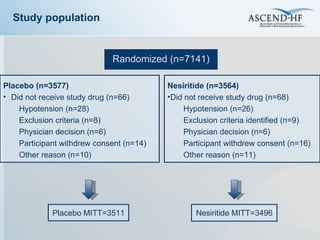
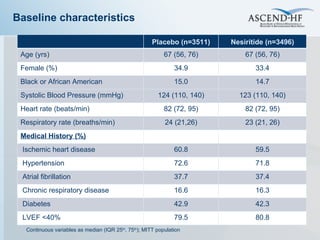
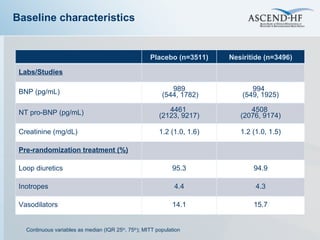
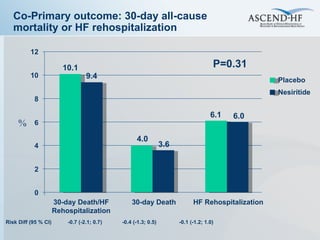
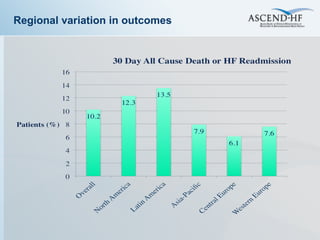
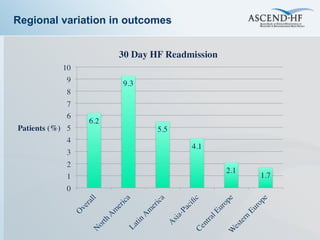
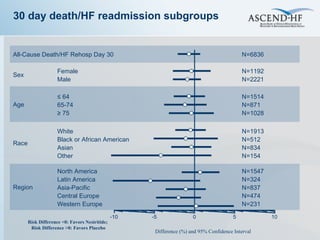
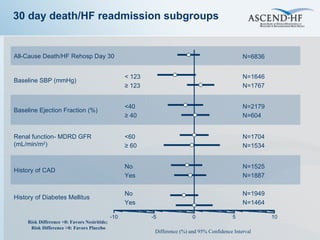
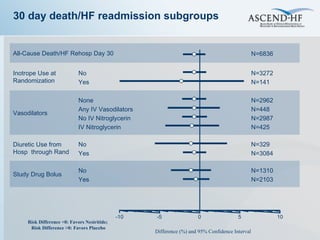
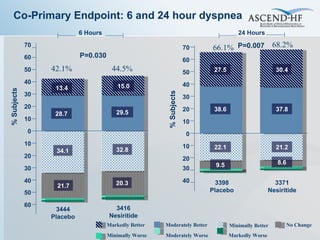
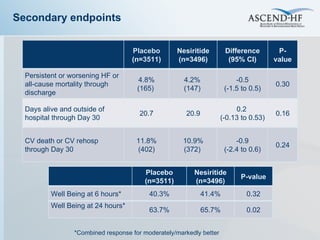
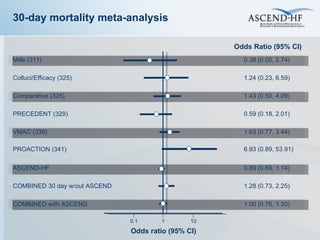
The document summarizes key information about acute heart failure, including epidemiology, pathophysiology, treatment approaches, and trial data. It describes the ASCEND-HF trial which investigated the effects of nesiritide vs placebo on outcomes in over 7,000 patients hospitalized for acute decompensated heart failure. The trial found no significant differences between nesiritide and placebo for its co-primary endpoints of 30-day mortality or heart failure rehospitalization and dyspnea relief at 6 and 24 hours.