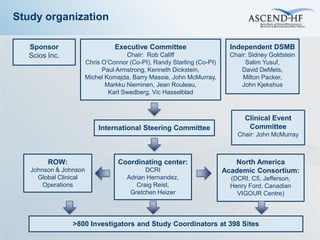
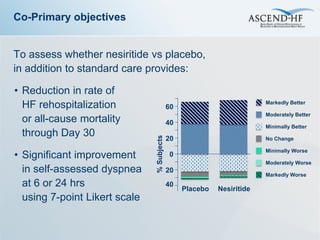
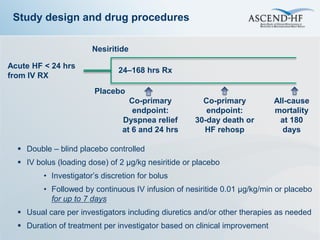
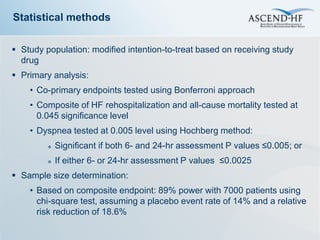
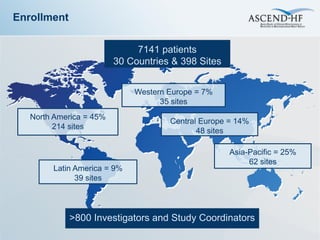
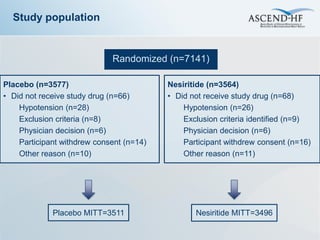
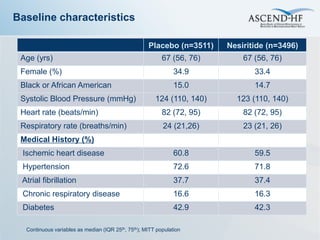
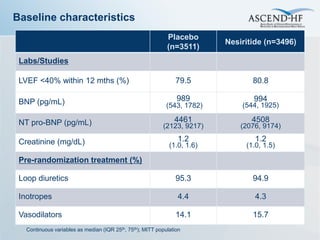
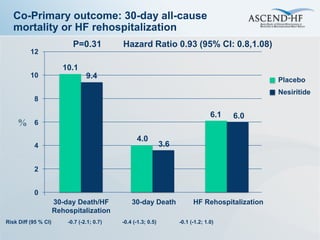
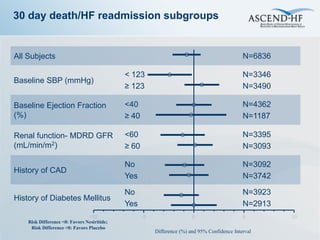
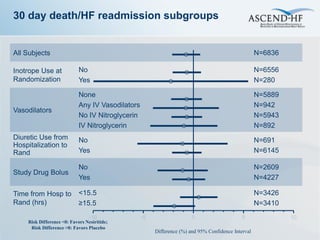
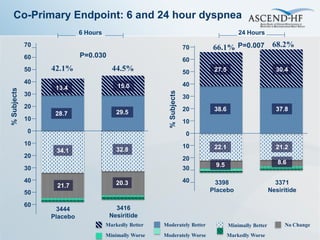
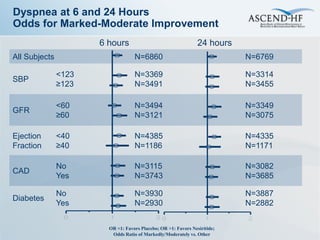
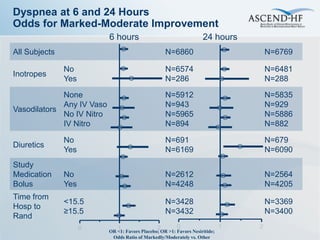
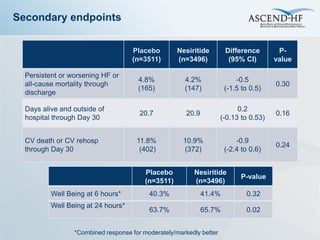
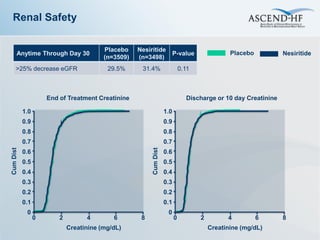
The ASCEND-HF trial aimed to assess the clinical effectiveness and safety of nesiritide compared to placebo for patients with acute decompensated heart failure. The randomized, double-blind, placebo-controlled trial enrolled over 7,000 patients across 30 countries. The co-primary endpoints were reduction in heart failure rehospitalization or mortality within 30 days and improvement in self-assessed dyspnea at 6 and 24 hours. Secondary outcomes included overall well-being, mortality, and safety. Baseline characteristics were well balanced between the nesiritide and placebo groups. Results of the trial could help definitively determine nesiritide's effects on important clinical outcomes in heart failure.