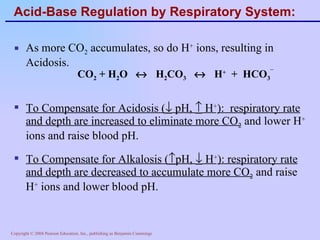
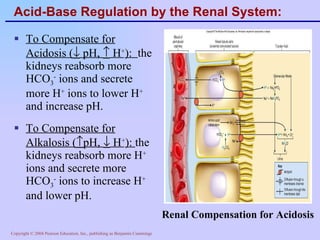
The document discusses acid-base balance and regulation in the human body. It defines normal blood pH as 7.35-7.45 and describes how the respiratory and renal systems work to regulate pH levels and compensate for acidosis or alkalosis. The respiratory system regulates pH by increasing or decreasing respiration to eliminate or retain carbon dioxide, while the renal system regulates pH by reabsorbing or secreting bicarbonate and hydrogen ions to compensate for acid-base imbalances.